Environmental Engineering Reference
In-Depth Information
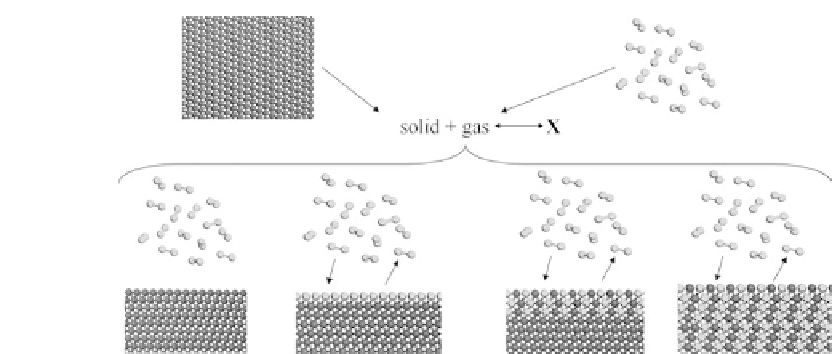
Figure 5.2
Schematic models of different solid/gas interface structures.
In order to evaluate which of these scenarios leads to the most stable interfacial
structure, we have to analyze the relation between the chemical potentials of both
reservoirs and the overall energy. Therefore, we begin with the Gibbs free energy of
the interface,
Þ¼
X
i
GT,
ð
p
fg
,
N
fg
N
i
m
i
(T, p
i
)
þ
Ag T,
ð
p
fg
Þ
(5
:
4)
which is the sum of the energy required to extract N
i
atoms or molecules of the ith
species from their reservoir with m
i
(T, p
i
) and the energy to build the interface with
contact area A.
The Gibbs free energy G and the chemical potentials include contributions from
the internal energy, vibrational free energy, and configurational entropy. Since most
relevant structures will have a low surface free energy, we obtain from (5.4) that
"
#
Þ
X
i
Þ
1
A
g T,
ð
p
fg
GT,
ð
p
fg
,
N
fg
N
i
m
i
(T, p
i
)
(5
:
5)
On the basis of this equation, we will now discuss different systems relevant to the
examples presented later.
5.2.2.1 Pure Solid in Contact with a Single- or Multi-Component
Environment
Considering the example of a Pt surface in contact and in thermo-
dynamic equilibrium with an oxygen atmosphere, the surface free energy (5.5)
becomes
g(T, p
O
2
, p
Pt
)
¼
1
A
[G(T, p
O
2
, p
Pt
, N
O
, N
Pt
)
N
Pt
m
Pt
(T, p
Pt
)
N
O
2
m
O
2
(T, p
O
2
)]
(5
:
6)







Search WWH ::

Custom Search