Environmental Engineering Reference
In-Depth Information
developed to study electrochemical interfaces (see Abruna [1991], Kolb [1996, 2002],
and Magnussen [2002], and references therein). Regarding theoretical studies, different
attempts have been made to model and understand the structure and properties
of systems under electrochemical conditions. An overview can be found in the reviews
of Schmickler [1996a, 1999], Koper et al. [2003], Koper [2004] and the papers of
Nazmutdinov and Shapnik [1996], Halley et al. [2000], Vassilev et al. [2001], Haftel
and Rosen [2003], Feng et al. [2005], Kitchin et al. [2004], Gunnarsson et al.
[2004], Taylor et al. [2006], and Jacob [2007a, b] and references therein. In these differ-
ent works, mainly experimental input, semi-empirical approaches, or rather simplified
models, have been used. The electrode potential has been either neglected or introduced
by charging the electrode surface or applying an external electric field. While most of
the theoretical studies have disregarded the electrode potential, some have attempted to
consider its influence on catalytic reactions. For instance, Nørskov's group [Kitchin
et al., 2004; Rossmeisl et al., 2006] studied the hydrogen evolution reaction (HER)
and oxygen reduction reaction (ORR) on different electrodes, whose Fermi energies
were shifted by the value of the electrode potential. Focusing more on the atomistic
structure of the interface, Neurock's group [Taylor et al., 2006] performed ab initio
molecular dynamics simulations on charged electrodes surrounded by water. For com-
pensation, a counter-charge was located at a certain distance from the electrode surface,
in an attempt to mimic the potential profile within the interfacial region.
While most theoretical studies have focused on electrochemical reactions by calcu-
lating the binding energies of particular adsorbates on the electrode, reaction barriers,
and reaction mechanisms, the influence of the morphology has often been underesti-
mated. Figure 5.1 schematically shows that there is a sensitive interplay between the
morphology of the system, determined by its structure and composition, and the ener-
getic of (electro)catalytic reactions. Furthermore, both are strongly influenced by the
environment, which, when taking part in the reaction, could even be reactive, and the
external parameters T, p/a, and electrostatic potential Df. Therefore, prior to investi-
gating detailed reactions, one has to understand the influence of these parameters and
the environment on the morphology of the electrochemical system.
Figure 5.1 Interplay between the morphology of a system, its environment and energetics,
and external parameters.






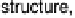


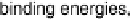











Search WWH ::

Custom Search