Environmental Engineering Reference
In-Depth Information
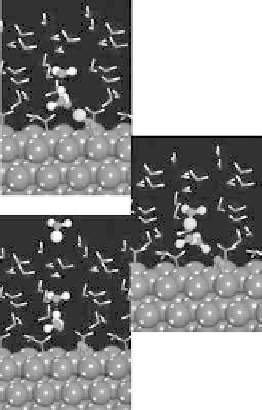
Figure 4.14 Free energy as a function of electrode potential for the species considered in the
reduction of O
2
to form OOH
. In the atomistic diagrams, purple coloring is used to illustrate
where the electron involved in reduction resides in each species. (See color insert.)
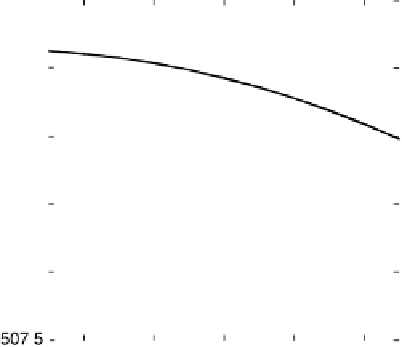
Figure 4.15 Overall barrier for O
2
reduction to OOH
over the Pt(111) surface as a function
of electrode potential. The inset shows the transition state for reduction at 1 V/NHE.


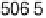

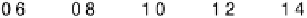
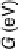

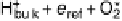



















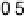







































Search WWH ::

Custom Search