Environmental Engineering Reference
In-Depth Information
to 0.62 eV in solution. The presence of water allowed for a direct heterolytic pathway
in which the (OC)O22H bond was activated to form a CO
2
2
and an H
þ
, which was
transferred through the local water molecules to form an H
3
O
þ
or H
5
O
2
þ
intermediate
as the CO22O bond is created. We reported that, in the solvated cell, the transition
states for CO
þ
OH coupling to COOH or with simultaneous O22H dissociation
over Pt are equivalent [Janik and Neurock, 2006]. The barrier to CO
þ
OH coupling
is reduced with respect to the UHV environment, owing to hydrogen bond stabiliz-
ation of the transition state (Fig. 4.13). The hydrogen bond distance between the
hydroxyl species and a nearby water molecule was reduced from 2.03
˚
in the initial
state to 1.80
˚
at the transition state, providing significant solvation stabilization to
lower the barrier with respect to the UHV environment.
Although the coupling of CO and OH is not a redox reaction, the changes in electrode
potential, and therefore the interfacial electric field, electrode Fermi level, and altered
water structure can alter the activation barrier. The calculation of potential dependent
barriers with the double-reference method requires the location of transition states with
varying system charges. Transition states are located using the climbing image nudged
elastic band method (CI-NEB) [Henkelman and Jonsson, 2000; Henkelman et al.,
2000; Mills et al., 1995], which optimizes a series of images between initial and final
states of an elementary reaction with the constraint of remaining spaced along the reaction
path and with a single image forced to maximize its energy along the reaction path. This
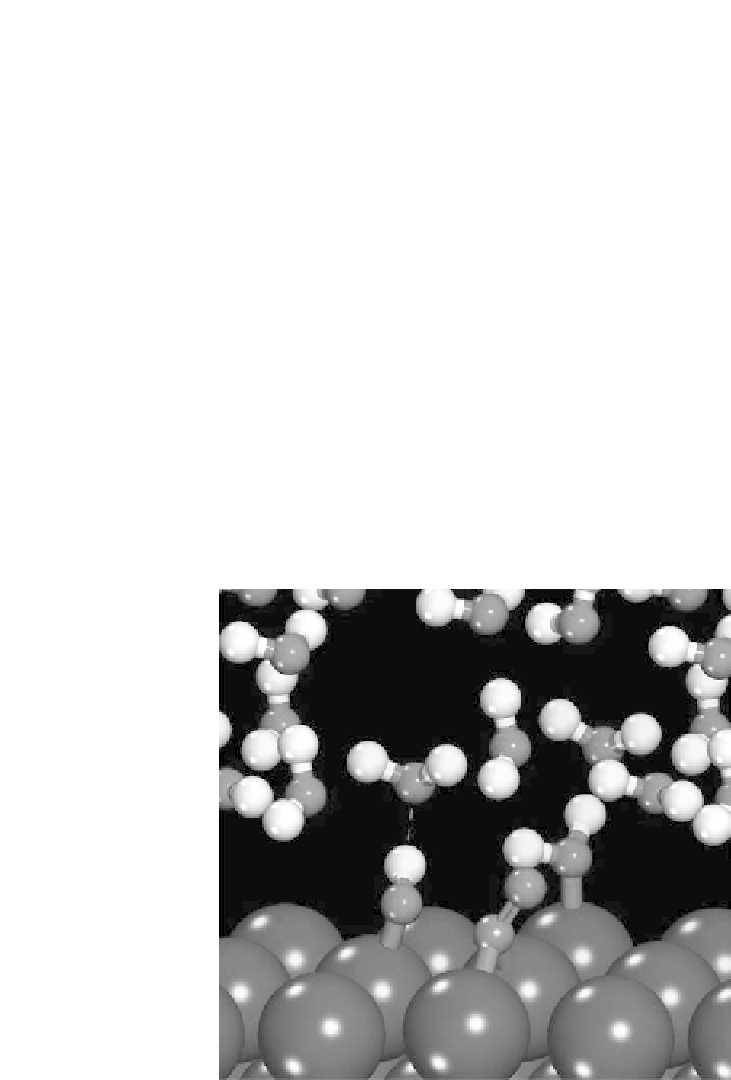
Figure 4.13 The transition state for CO
þ
OH coupling to form COOH on the Pt(111) sur-
face. The transition state is stabilized in the aqueous model system through hydrogen bonding
between the hydroxyl species and a nearby water molecule [Janik and Neurock, 2006].






Search WWH ::

Custom Search