Environmental Engineering Reference
In-Depth Information
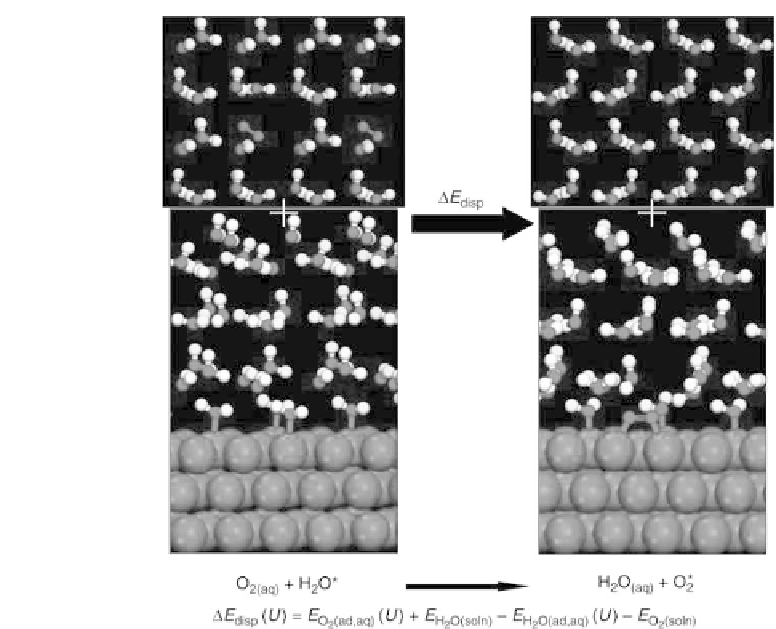
Figure 4.9 Illustration of the structures considered for adsorbing an oxygen molecule from
solution to the electrode surface, displacing a water molecule from the surface to solution.
The energy associated with the adsorption of O
2
at a Pt(111) electrode surface is
relevant to the oxygen reduction reaction (ORR) occurring at the cathode of a
PEMFC. Alloying a second metal with Pt can increase the rate of the ORR by weak-
ening the binding of oxygen-containing species and limiting hydroxyl poisoning of
the surface. However, in sacrificing strong oxygen binding, the ability of molecular
oxygen to compete with water molecules for metal surface sites may be decreased
as well. The relative binding preferences of molecular oxygen and water may be
potential-dependent. The adsorption of water occurs primarily through the donation
of electron density from the 1b
1
lone pair of electrons on the oxygen atom into the
unoccupied d
z
2
orbital on the metal [Henderson, 2002], and binding energies may
be expected to increase in strength as the electrode potential is increased (with respect
to an NHE), or the workfunction of the metal is increased. Molecular oxygen, on the
other hand, binds through both donation from molecular oxygen into the metal and
back-donation from the metal into the 2p
orbital on oxygen. Oxygen picks up elec-
tron density from the metal through back-donation, resulting in an elongation of the
O22O bond when adsorbed to the surface. Both cluster [Hyman and Medlin, 2005;
Panchenko et al., 2004] and periodic [Hyman and Medlin, 2005] DFT models of






Search WWH ::

Custom Search