Environmental Engineering Reference
In-Depth Information
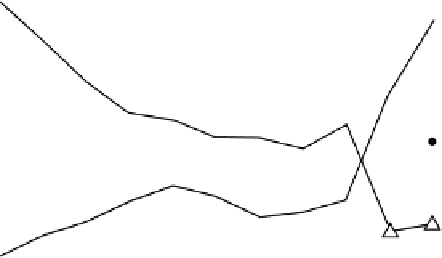
Figure 4.7 The correlation of metal atom charge with the metal - oxygen bond length in the
water adsorption as a function of the total system charging in a representative copper - water
electrode/electrolyte interface. Open triangles: height of the adsorbed oxygen above the surface
plane (either as H
2
O, OH, or O). Filled triangles: integrated charge density within a sphere of
radius 1.45
˚
about the coordinated copper atom directly coordinated to the dissociating
water molecule. Points a-g correspond to the following: a - b, water migration from atop to
bridge; b-c, dissociation to form OH and H
þ
; c-d, migration of OH to 3-fold coordination;
d-e, dissociation of OH to O
þ
H
þ
; e-f, gradual formation of oxide; f-g, oxide formation
[Taylor, 2009b].
moved up or down [Rossmeisl et al., 2006]. Hence, plots of the dissociation reaction
energy are linear in the potential with only slight deviations due to these bonding
effects. This effect is demonstrated by the energy versus potential correlation in
Fig. 4.8 for water dissociation over Pt(111), in which the deviation of the curve
from linearity is shown by the variance of the solid lines (full model) from the
dashed lines (linear term only model). Inclusion of the hydrogen chemical potential
(i.e., the pH) can be incorporated easily into the thermodynamic model using the
Nernst equation. The production of OH thus occurs at more positive potentials
when the pH is lowered, whereas the production of H from H
2
O occurs at more nega-
tive potentials when the pH is raised. An extensive survey of DFT predictions for
electrochemical water dissociation has been made for a series of transition and
noble metals, with good qualitative and semiquantitative agreement for the known
double-layer regions for these metals [Taylor et al., 2007b]. It is important to be
aware of the state and reactivity of water on the surface when exploring other chemistry
that occurs in the presence of an aqueous environment (i.e., fuel cell activity). This
synergism is discussed more fully in the following sections.
The results found here indicate that although the changes in potential can markedly
influence oxidation reaction energy through altering the energy of the electron
product, differences in the interactions of the reactant (water) and product (hydroxyl)
species with the interfacial electric field appear to have only a small effect on the






























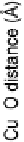

















Search WWH ::

Custom Search