Environmental Engineering Reference
In-Depth Information
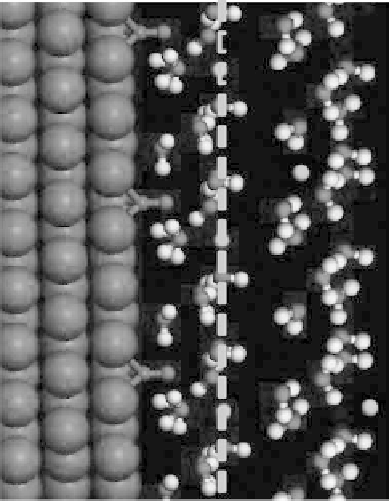
Figure 4.3 Schematic diagram of the electrochemical metal2aqueous interface, with the
electrode, inner layer, diffuse layer, outer Helmholtz plane (OHP), and inner-layer thickness
x
il
indicated.
in the half-cell model system while considering chemical transformations that alter the
electronic structure. Current ab initio quantum mechanical methods are canonical,
based on constant electron systems. Electrochemical half-cells, however, are based
on constant chemical potential (instantaneously maintained by non-faradaic current
from the potentiostat); this grand canonical ensemble is not feasible with current elec-
tronic structure methods. Despite such challenges, a variety and hierarchy of compu-
tational approaches to modeling the surface potential (and potential drop across the
inner layer) have been developed [Anderson, 1981; Dominguez-Ariza et al., 2004;
Halley et al., 1985; Head-Gordon and Tully, 1993].
The recent interest in the exploration of electrocatalytic phenomena from first prin-
ciples can be traced to the early cluster calculations of Anderson [1990] and Anderson
and Debnath [1983]. These studies considered the interaction of adsorbates with
model metal clusters and related the potential to the electronegativity determined as
the average of the ionization potential and electron affinity—quantities that are
easily obtained from molecular orbital calculations. In some iterations of this
model, changes in reaction chemistry induced by the electrochemical environment

















Search WWH ::

Custom Search