Environmental Engineering Reference
In-Depth Information
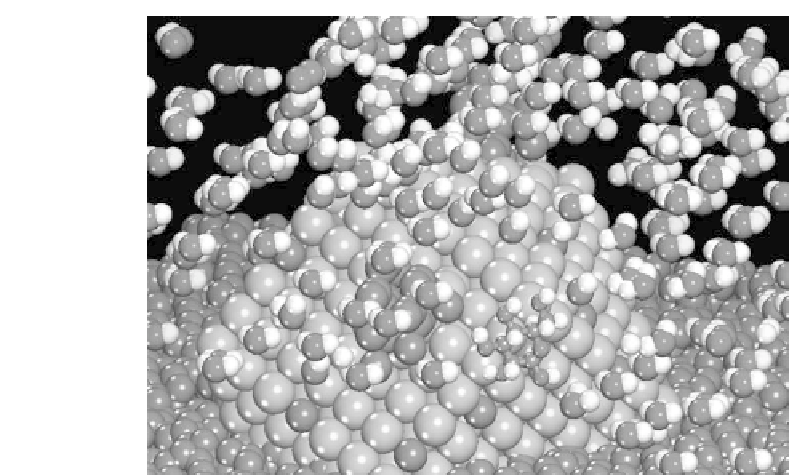
Figure 4.1 Schematic of the atomic structure of the active three-phase interface between the
metal particle that catalyzes the reaction, the carbon support necessary to conduct electrons, and
the polymer electrolyte and solution necessary to conduct protons for electrocatalytic systems.
here, including the influence of metal particle size and morphology effects, the role of
metal defect sites (i.e., corner, edge, and kink sites), the influence of the support,
alloying effects, and the influence of actual operating conditions that control the
actual surface coverages. In addition, the unique electrocatalytic reaction environment
of the “buried interface” presents a number of new issues. These include the influences
of the following factors on the catalytic processes that occur at the three-phase interface:
†
solution or humidity
†
the electrochemical potential
†
polymer electrolyte
†
proton transfer
†
electron transfer
While these features create a number of challenges in establishing fundamental
insights for experiment as well as for theory and simulation, they also represent
important opportunities, as they are the features by which one can control the
design of new materials.
In this chapter, we will focus on some of the recent developments in understanding
the influence of solution and electrochemical conditions over model single-crystal sur-
faces. Specifically, we will review work applying electronic structure methods to probe
electrocatalytic mechanisms occurring at this complex interface.






Search WWH ::

Custom Search