Environmental Engineering Reference
In-Depth Information
RhRe, while intriguing, would likely be of too high a cost to be of practical interest.
BiPt, in contrast, is worth further consideration. From a practical point of view, Bi is
a very cheap metal, suggesting that some cost savings might be realized by using
BiPt (as opposed to pure Pt) as an HER catalyst. More importantly, however, BiPt pre-
sents unusual properties from a fundamental scientific point of view. The novelty here
is related to the stark contrast between its two constituent elements; pure Pt exhibits
high HER activity, while pure Bi is not active at all. A surface alloy formed from
these two elements, however, yields a material predicted by the calculations to have
an activity comparable to, or even better than, pure Pt. This counterintuitive result
suggest that BiPt is worthy of further experimental study and validation.
3.6.8 Experimental Results
Given that a BiPt surface alloy, identified by our combinatorial, computational search
procedure, appears to be a novel and interesting HER candidate, we set out to synthesize
and test it experimentally. A three-step approach was used: (i) an initial Pt film was
electrodeposited onto an inert support, (ii) a sub-monolayer of Bi was spontaneously
deposited onto the film by Bi-UPD, forming adsorbed Bi adatoms, and (iii) the
Pt - Bi precursor film was annealed to form the surface alloy (see [Greeley et al.,
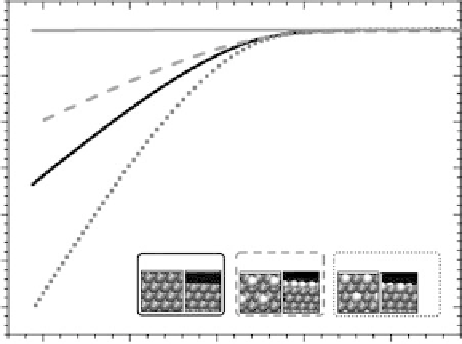
2006a] for details). Current - voltage measurements of the Pt film, the Bi adatoms
(denoted Pt - Bi
ir
), and the annealed surface alloy are plotted in Fig. 3.21.
Immediately after Bi adatom deposition, the measured activity of the Pt - Bi
ir
sample
is considerably less than that of the initial Pt film—the Bi
ir
blocks Pt sites and poisons
the surface for hydrogen evolution [Markovic and Ross, 2002]. However, after the
Figure 3.21 Hydrogen evolution after each stage of BiPt surface alloy synthesis. (a) Pt film
after deposition and anneal; (b) immediately after Bi-UPD; (c) after second anneal to form
the BiPt surface alloy. Adapted from [Greeley et al., 2006a]; see this reference for more details.





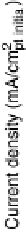
























Search WWH ::

Custom Search