Environmental Engineering Reference
In-Depth Information
The combination of activity and stability considerations described above identifies
approximately 30 surface alloys that could be robust catalysts for the HER [Greeley
and Nørskov, 2007]. To further refine this list of candidates, we perform a Pareto-
optimal analysis of the aforementioned activity and stability features. This analysis
is implemented by plotting the most negative of the free energy transformation
values determined for each alloy (i.e., the most pessimistic of the stability criteria
for each alloy described above, which might vary from alloy to alloy) against the
absolute magnitude of DG
H
. In essence, we are simply putting the stability and activity
criteria for the various alloys on a single plot. The stability considerations immediately
eliminate a large number of alloys from consideration; although many alloys have high
predicted HER activity, only a small fraction are predicted to be both active and stable
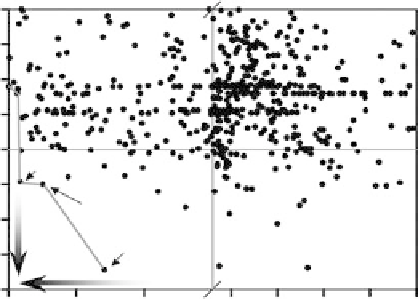
in acidic HER environments (Fig. 3.20).
Using the raw data in Fig. 3.20, we can identify the Pareto-optimal set for the HER
activity/stability criteria. This set represents the best possible compromise between
activity and stability criteria for the surface alloys that we have considered; the alloys
in the set are, thus, logical choices for further consideration. The presence of pure Pt
on the Pareto-optimal set is, in effect, a “sanity check” for our computational screening
procedure. Pt is well known to be the most active and stable pure metal for the HER in
acidic conditions. The alloys seen on the Pareto-optimal set include RhRe and BiPt.
Figure 3.20 Pareto-optimal plot of stability and activity of surface alloys for the hydrogen
evolution reaction (HER). The stabilization free energy can be thought of as a free energy of
formation for the surface alloys; the stability of the alloys with respect to various reconstruc-
tive/deactivating processes (including surface segregation, island formation, water splitting/
oxygen adsorption, and metal dissolution) is evaluated for each alloy, and the most pessimistic
such energy (i.e., the energy that would give the maximum probability that the alloy would
destabilize) is plotted. The Pareto-optimal line indicates the best possible compromise between
activity and stability, but, given the simplicity of our model, other alloys could certainly be
worth considering for use as HER catalysts; the alloys in the lower left quadrant, in particular,
are promising. For the labeled points, a single element indicates a pure metal. For the bimetallic
alloys, the solute is listed first; the solute coverages are
3
ML (BiPt) and 1 ML (RhRe). Adapted
from [Greeley et al., 2006a]; see this reference for more details.

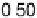
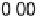

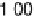
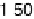



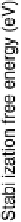
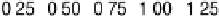


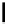














Search WWH ::

Custom Search