Information Technology Reference
In-Depth Information
Figure 3.1
Mixers/splitters.
1
MIX1
4
2
3
Figure 3.2
Mixer block.
flow rate and enthalpy/mole of stream
i
, the material balance and energy balance are
F
4
=
F
1
+
F
1
+
F
3
(3.1)
h
4
F
4
=
h
1
F
1
+
h
2
F
2
+
h
3
F
3
(3.2)
When energy balances are selected, it is necessary to specify the outlet stream's
pressure or the pressure drop across the block. If no pressure is specified, the block
uses the lowest pressure of the feed streams. Additionally, the allowable phases of the
output stream must also be specified. Since the output stream is of known composition
and pressure and its molar enthalpy is calculated using equation (3.2), its state is
defined, and therefore an adiabatic flash yields its temperature.
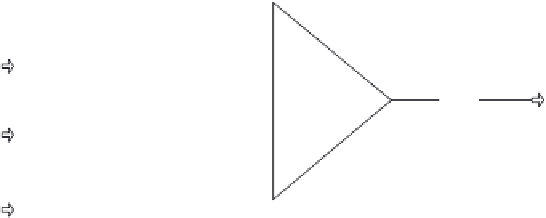
3.1.2 Fsplit Block
The Fsplit block is designed to divide a single stream or a mixture of streams of
known state (i.e., flow rate, composition temperature, and pressure) into an arbitrary
number of product streams with identical states. Note that since the state is known,
the enthalpy/mole is calculable. A choice of product stream split fraction (based on
the sum of the flow rates of the feed streams), mass or molar flow rate, volumetric
flow rate, or flow rate of a component may be made. If there are
n
product streams,
specifications for any
n
−
1 streams must be made. Figure 3.3 shows an example of
an Fsplit block.
When the model is executed, all the feed streams are mixed and the combined flow
rate, composition, and molar enthalpy is computed. It is important to note that any
of a block's product specifications can be converted to product stream split fractions.
This provides the basis for the describing equations of the model. For example, if the
molar flow rate of component
i
in stream
j
,
p
i
, is specified, the split fraction
α
i
can