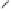Information Technology Reference
In-Depth Information
ETAZL
B2
B1
ETAZH
CRUDETOH
ETH
TOL
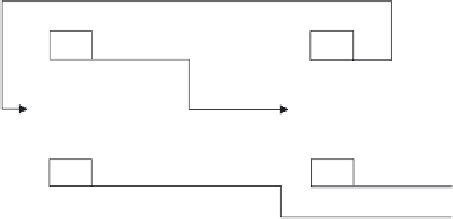
Figure 14.3
Homogeneous azeotropic distillation flowsheet.
14.2 HOMOGENEOUS AZEOTROPIC DISTILLATION
Figure 14.3 shows an example of the configuration of two distillation columns set up for
homogeneous azeotropic distillation. The intent of the separation is to produce nearly
pure ethanol from a mixture of ethanol and toluene whose composition is below the
azeotrope's composition. The arrangement of the two columns takes advantage of the
difference in azeotrope composition at different pressures. To facilitate the following
example, Aspen Plus's stored Wilson equation binary parameters, which are shown
in Table 14.1 are used to estimate the composition of the azeotrope as a function of
pressure. If these were to be used for an industrial application, the results would need
to be checked against experimental data.
The basic idea of the separation is explained with reference to Figure 14.3. A
simple material balance shows that the larger the pressure differential between the two
azeotropes, the smaller the recycle flow ETAZL. For illustration purposes, the higher-
pressure column, B1, is selected to operate at 760 mmHg and the lower-pressure
column, B2, at 50 mmHg. The stream ETAZH's composition is approximately 0.815
mole fraction ethanol and feeds the column B2. The stream ETAZL's composition
is approximately 0.685 mole fraction ethanol and is a second feed to column B1.
The bottoms product of column B2 is nearly pure ethanol, and the bottoms product
of column B1 is nearly pure toluene. Other pressures can surely be selected with an
analysis of the trade-off between recycle flow and associated operating costs against
the costs associated with purchase and installation of the columns. An initial estimate
of the design of the two columns can easily be done using the McCabe -Thiele method
on both columns. The low-pressure column can be analyzed with the toluene as the
more volatile component.
When such a design is undertaken, the following procedure is recommended:
1. Locate a source of experimental data and fit it to an activity coefficient equation.
2. Determine the azeotrope composition as a function of pressure.
TABLE 14.1 Ethanol-Toluene Azeotrope at Various Pressures
Pressure (mmHg)
50
100
400
760
1420
Mole fraction ethanol
0.685
0.715
0.77
0.815
0.85