Information Technology Reference
In-Depth Information
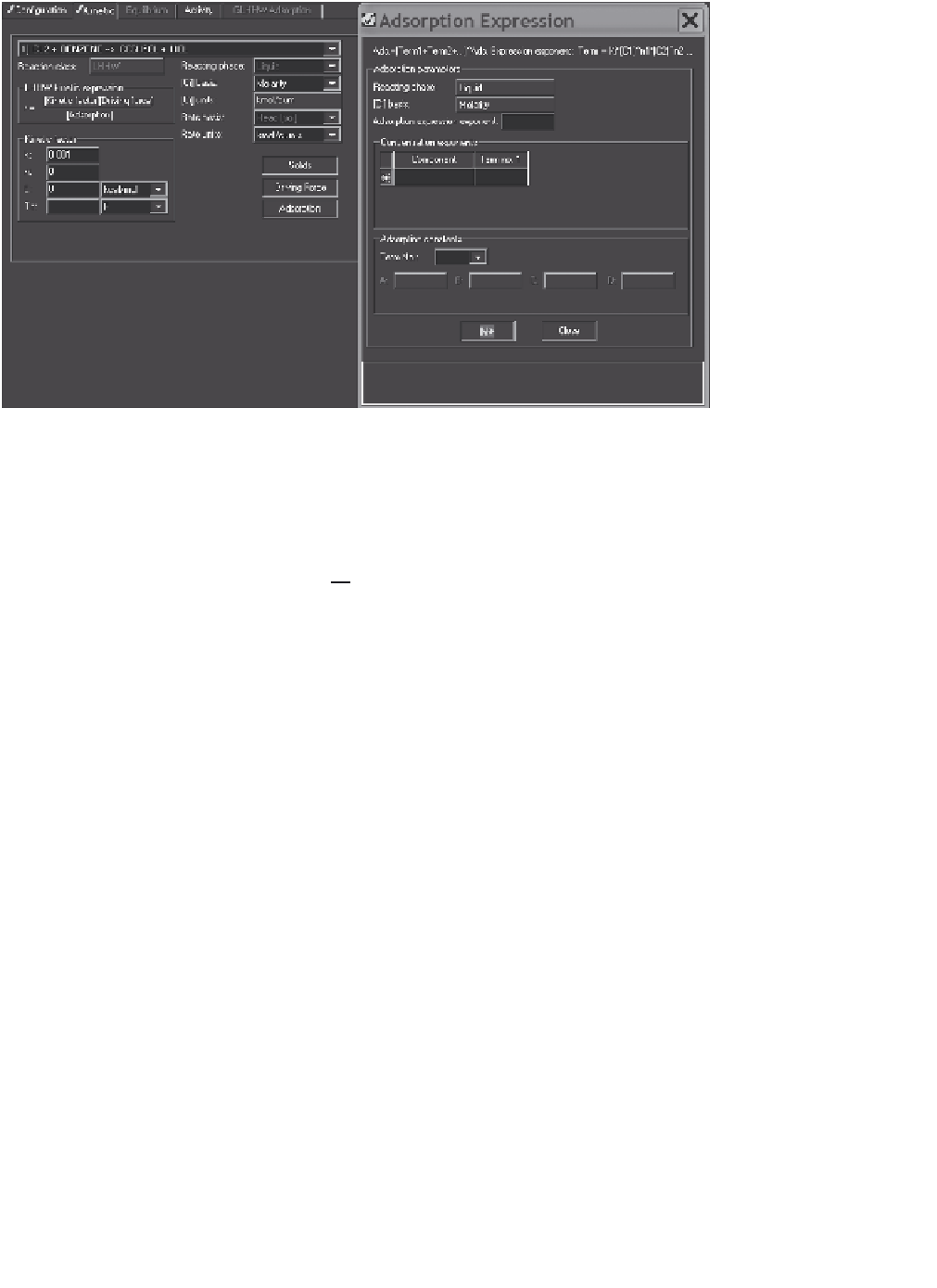
Figure 10.14
LHHW kinetic and adsorption expressions.
where
i
and
j
are component indices,
v
is a concentration exponent, and
m
is an
adsorption expression. The
K
i
are adsorption equilibrium constants, given by
B
i
T
+
C
i
ln
T
ln
K
i
=
A
i
+
+
D
i
T
(10.15)
where
T
is temperature in kelvin and
A, B, C
,and
D
are user-provided parameters.
An example of the reaction setup is given in Figure 10.14. When the adsorption
button is pushed, the right-hand display appears and facilitates the entry of adsorption
data.
10.5.4 Generalized-Langmuir-Hinshelwood-Hougen-Watson Class
The GLHHW class is designed to enable the user to prepare a customized adsorp-
tion term, which is best illustrated by Figure 10.15, which is displayed when the
GLHHW tab is selected. Note the equation near the bottom of the figure which
begins with Ads.
=
. This is the complete denominator: that is, the adsorption term
in the rate equation. The equation is composed of terms each containing a product
of
K
i
[equation (10.13)] and exponentiated component concentration terms. The exact
details of the resulting combination can be seen from the artificial example shown in
Figure 10.15. Note that the complete series of products is raised to the power of the
adsorption exponent.
10.6 RCSTR BLOCK
The basic ideas describing the analysis of steady-state continuously stirred reactors
is well described by Levenspiel (1962). A key element of the analysis is that the