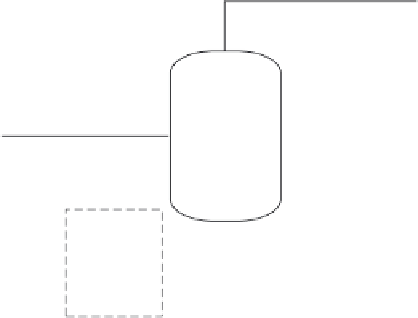
Information Technology Reference
In-Depth Information
Vapor, V, v
Flash2
P
Feed, F, f
Heat, Q
Liquid, L, l
Figure 7.1
Flash2 model.
variables by an equation such as
v
i
j
=
1
v
j
y
i
=
(7.1)
where
y
i
is the mole fraction of component
i
in the vapor.
The applicable material balances are
n
componential equations such as
f
i
−
v
i
−
l
i
=
0
(7.2)
or alternatively,
n
−
1 equations such as equation (7.2) and one overall material balance
given by
F
−
V
−
L
=
0
(7.3)
Additionally,
n
equilibrium equations which describe the equality of the fugacities
of components in each phase are required. When the liquid fugacity is represented by
an equation of state, where
φ
i
are the fugacity coefficients of component
i
in
the liquid and vapor phases, respectively, the result is
i
and
φ
V
i
L
i
y
i
φ
−
x
i
φ
=
0
(7.4a)
When the liquid fugacity is represented by an activity coefficient equation, where
γ
i
is the activity coefficient of component
i
and the vapor phase is represented by an
equation of state
V
i
P
− γ
i
x
i
p
i
y
i
φ
=
0
(7.4b)
where
p
i
is the vapor pressure of component
i
, results. For the sake of simplicity, the
Poynting correction (see Prausnitz et al., 1999), which has a contribution only for very
light components, has been omitted.