Chemistry Reference
In-Depth Information
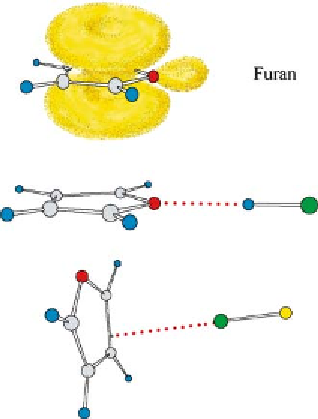
Fig. 18
The n-pair/
π
-pair model of furan together with the experimental geometries of
furan
HCl, which has a planar geometry of C
2
v
sym-
metry with HCl lying along the C
2
axis, clearly obeys rule 3 but the observed face-on
arrangement for furan
···
HCl and furan
···
ClF. Furan
···
ClF demonstrates that rule 3 is violated in this case. See Fig. 1
forkeytothecolourcodingofatoms
···
-pair donors can be understood
by considering the electric charge distributions for the series of heterocyclic
molecules pyridine, furan and thiophene. A convenient tabulation of the mo-
lecular electric dipole and quadrupole moments of these compounds is given
in ref. [19]. The electric dipole moment decreases along the series, an obser-
vation which has been interpreted as indicating a progressive withdrawal of
the n-pair on the heteroatom into the ring. On the other hand, the magnitude
of out-of-plane component,
Q
cc
, of the electric quadrupole moment, which
is a measure of the extension of the
Thebehaviourofthen-pair/aromatic
π
cloud above and below the molecular
plane, increases along this series. Thus, thiophene is the member of the se-
ries most likely to violate rule 3 and pyridine the least likely. Certainly, both
the HCl and ClF complexes of thiophene are of the
π
π
-type. The complexes
pyridine
HX, for X = F [170], Cl [171] and Br [172] are all of the n-type,
with HX lying along the C
2
axis and forming a strong hydrogen bond to the
heteroatom. Furan is the intermediate case and whether the n- or
···
-electron
pairs define the geometry of the complex is evidently sensitive to the pre-
cise nature of the electron acceptor. Thus, furan
π
···
HCl is an n-pair complex
while furan
HBr [173]
also has the face-on conformation, so there is a changeover between X = Cl
and Br in the furan complexes.
···
ClF has a
π
-type interaction. Interestingly, furan
···
Search WWH ::

Custom Search