Chemistry Reference
In-Depth Information
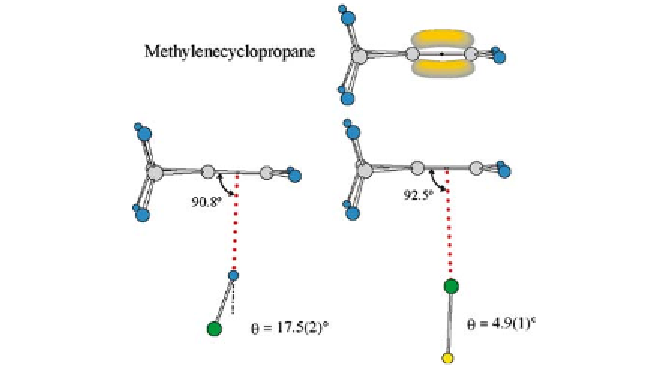
Fig. 16
The experimental geometries of methylenecyclopropane
···
HCl and methylene-
cyclopropane
-electron model for the Lewis base is also
shown. The angles C-
∗· · ·
HandC-
∗···
Cl, respectively, where
∗
is the centre of the C - C
double bond, are both close to 90
◦
, as required by rule 2. The halogen bond again exhibits
a smaller non-linearity
···
ClF, drawn to scale. The
π
θ
than the hydrogen bond. See Fig. 1 for key to the colour coding
of atoms
symmetry that the hydrogen bond is significantly non-linear while the corres-
ponding halogen bond is not. We shall return later (Sect. 6) to this important
difference between the two types of intermolecular bond. Other complexes of
methylenecyclopropane with HX (X = F [144] and Br [145]) have geometries
similar to that for X = Cl.
3.3
Angular Geometries of B···ClF and B···HCl
in Which B is a Mixed n-Pair/π-Pair Donor
According to rule 3, if a Lewis base B carries both non-bonding and
-
bonding electron pairs, the n-pairs are definitive of the angular geometry.
There are many examples of simple Lewis bases B that can in principle act
as either n- or
π
-electron pair donors. These include CO, HCN, H
2
CO, furan,
thiophene, pyridine, etc. We note that, for convenience, we considered H
2
CO
earlier as an example of a Lewis base carrying a pair of equivalent n-pairs
and ignored the
π
ClF [79] are
examples that obey rule 3. The complexes HX with carbon monoxide when
X = F [146], Cl [147], Br [148], and I [149] have all been investigated through
their rotational spectra. Each is linear, with the order of the atoms OC
π
pair. In fact, H
2
CO
···
HCl [121] and H
2
CO
···
HX
in the lowest energy conformer, so that the n-pair on the C atom takes prece-
dence over the
···
π
pairs (and indeed the n-pair on O), as predicted by rule 3.
Search WWH ::

Custom Search