Chemistry Reference
In-Depth Information
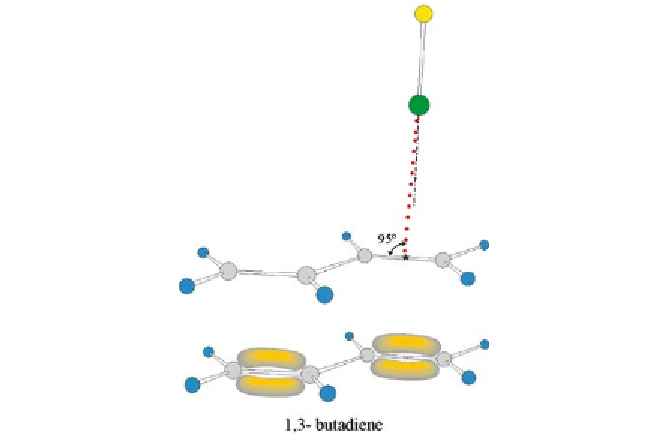
Fig. 13
The experimental geometry of 1,3-butadiene
···
ClF drawn to scale and the
π
-
electron model of 1,3-butadiene. The geometry of 1,3-butadiene
···
HCl is not yet available
forcomparison.SeeFig.1forkeytothecolourcodingofatoms
electric resonance spectroscopy and pulsed-jet, Fourier-transform microwave
spectroscopy) involving supersonic expansion of gas mixtures of benzene and
HX in argon. In each case, only information about the vibrational ground
state is available. Benzene
ClF also has a symmetric top-type spectrum
but exhibits evidence of non-rigid-rotor behaviour [80]. The ground-state
spectrum is accompanied by a single vibrational satellite spectrum which is
presumably associated with a low-energy vibrationally excited state, given
the low effective temperature of the experiment. A possible interpretation of
these observations for benzene
···
ClF is that the geometry of the complex is
asshowninFig.14,thatis,inthezero-pointstate,theClFsubunitexecutes
the motion defined by the angle
···
= 0
◦
)
φ
,withaPEmaximumattheC
6
v
(
φ
δ
+
Cl of the ClF subunit interacts
conformation. Thus, the electrophilic end
with the
φ
coordinate, as indicated, encompassing the six carbon atoms. This path pre-
sumably corresponds to a potential energy minimum relative to a maximum
at the C
6
v
(
π
-electron density as it traces out the nearly circular path in the
= 0
◦
) conformation but is itself likely to present small maxima at
the carbon atoms.
It is possible that the complexes benzene
φ
HX can be described in a simi-
lar way, but in the absence of any observed non-rigid-rotor behaviour or a vi-
brational satellite spectrum, it is not possible to distinguish between a strictly
C
6
v
equilibrium geometry and one of the type observed for benzene
···
ClF. In
either case, the vibrational wavefunctions will have C
6
v
symmetry, however.
···
Search WWH ::

Custom Search