Chemistry Reference
In-Depth Information
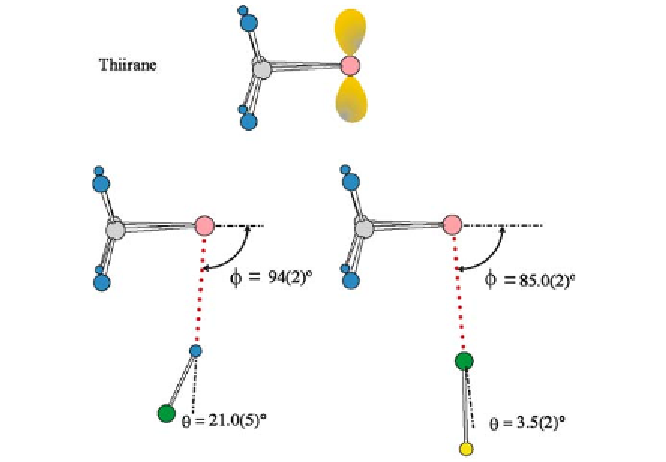
Fig. 9
The experimentally determined geometries of thiirane
···
HCl and thiirane
···
ClF
φ
drawn to scale. The n-pair model of thiirane is shown for comparison. The angle
is
slightly different in the two complexes for reasons discussed in [69]. The non-linearity
θ
of the hydrogen bond is again greater than that of the halogen bond. See Fig. 1 for key to
thecolourcodingofatoms
slightly smaller angle in the case of (CH
2
)
2
S
···
ClF is discussed in [69]. It is
clear that the hydrogen bond in (CH
2
)
2
S
···
HCl deviates significantly from
= 21.0(5)
◦
]whilethehalogenbondin(CH
2
)
2
S
linearity [
φ
···
ClF is close to
=3.5(2)
◦
]. The hydrogen bonds in the complexes (CH
2
)
2
S
linear[
φ
···
HF [124]
and (CH
2
)
2
S
···
HBr [28, 125] are also significantly non-linear.
3.1.3
B Carries Two Inequivalent n-Pairs
Sulfur dioxide is an example of a simple Lewis base that carries two sets of in-
equivalent n-pairs, one set on each O atom. The n-pair model (in which the
π
bonding pairs are not drawn and are ignored here) is shown in Fig. 10. The
geometries of SO
2
ClF [70]
have all been determined from investigations of their rotational spectra. Each
molecule is planar and belongs to the C
S
point group. Scale drawings for
SO
2
···
···
HF [126, 127], SO
2
···
HCl [28, 126] and SO
2
···
HCl and SO
2
···
ClF are displayed in Fig. 10.
We note that the HCl and ClF molecules attach, approximately at least,
along the axis of the
cis
n-pair, as required by rule 1, with angles
of 143.0(1)
◦
and 131.9(6)
◦
, respectively, although the former value may be influenced by
φ
Search WWH ::

Custom Search