Chemistry Reference
In-Depth Information
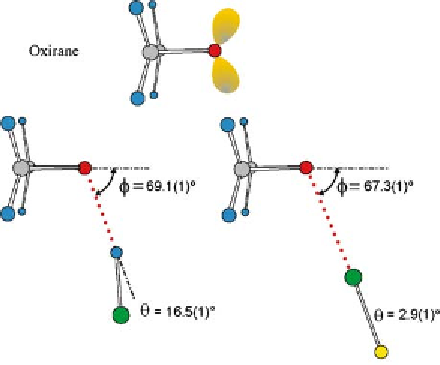
ing structure must also reproduce this angle. The geometry of (CH
2
)
2
O
···
HCl
so obtained [28, 120] is shown in Fig. 6.
The angle
is
the angle O-H-Cl, as indicated. Also shown in Fig. 6 is the geometry simi-
larly determined for the halogen-bond analogue of (CH
2
)
2
O
θ
defines the non-linearity of the hydrogen bond and
φ
···
HCl, namely
(CH
2
)
2
O
···
ClF [67]. We note immediately a striking similarity between the
of these two complexes [69.1(1)
◦
and 67.3(1)
◦
, respectively], a re-
sult that can be understood on the basis of rule 1 if the oxygen atom of
oxirane carries two equivalent n-pairs, as drawn schematically in Fig. 6.
By contrast, there is a significant difference in the non-linearities [
angles
φ
θ
=
16.5(1)
◦
and 2.9(1)
◦
]ofthehydrogenbondO
···
H - Cl and the halogen bond
O
Cl - F in the two complexes. We shall see that this relationship between
(CH
2
)
2
O
···
···
HCl and (CH
2
)
2
O
···
ClF is an example of a common property of
the two series B
ClF and, moreover, that the propensity to be
non-linear is an important characteristic of the hydrogen bond.
Other Lewis bases in which the electron donor atom Z carries two equiva-
lent n-pairs and which form complexes of C
S
symmetry with HCl and ClF
have been investigated by the same approach. The resulting geometries when
B is formaldehyde are shown, together with the conventional n-pair model
of CH
2
O, in Fig. 7. The angle
···
HCl and B
···
φ
is virtually identical in H
2
CO
···
HCl [121]
and H
2
CO
ClF [79] and is close to that expected from the n-pair model in
which the angle between the n-pairs is
···
120
◦
. The hydrogen bond again de-
∼
= 20.3(8)
◦
]buttheO
viates significantly from linearity [
θ
···
Cl - Fsystemis
=3.2(7)
◦
].
essentially collinear [
θ
Fig. 6
ClF
drawn to scale. The n-pair model of oxirane is shown for comparison. While the angle
φ
is similar in both complexes, the non-linearity
θ
of the hydrogen bond is much greater
than that of the halogen bond. See Fig. 1 for key to the colour coding of atoms
The experimentally determined geometries of oxirane
···
HCl and oxirane
···
Search WWH ::

Custom Search