Chemistry Reference
In-Depth Information
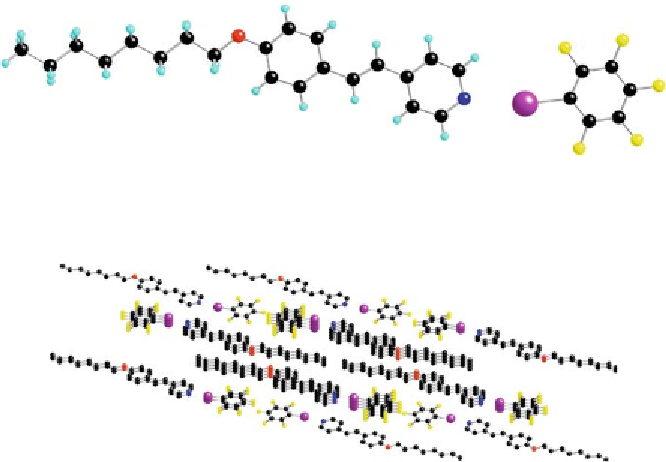
Fig. 12
Molecular structure of the complex between 4-octyloxystilbazole and iodopentaflu-
orobenzene. Reproduced by kind permission of the American Chemical Society
Fig. 13
One projection of the packing of
13
-8 - hydrogen atoms omitted for clarity
backed up by the lack of any evidence for interactions between stilbazole and
hexafluorobenzene [41]. In relation to this, it was observed that while the
starting stilbazole was colourless, the halogen-bonded complex was slightly
coloured. Such an observation is entirely consistent with a charge-transfer in-
teraction at nitrogen and parallels the red shift observed in the VT electronic
spectra for stilbazole and 2,4-dintrophenol (vide supra) [37, 38].
The liquid crystal properties of the complexes were characterised using po-
larised optical microscopy and showed a nematic phase for
n
=4and6and
a SmA phase for
n
= 6, 8, 10 and 12. The mesophases were monotropic for
n
= 4 and 6 and enantiotropic for the others; the progression from a nematic
phase for shorter chain lengths to SmA at longer chain lengths is quite typical
for simple, polar mesogens.
Halogen bonding is also observed with electron-poor bromides, and so
attempts were made to form complexes between stilbazole and
bromo
penta-
fluorobenzene. We were never able to find evidence that such a complex
formed and indeed, heating crystallised samples only reproduced the ther-
mal behaviour of the stilbazoles themselves. Thus, any halogen bonding is
supposed weak (there was no observable colour change in the stilbazole) and
unable to sustain the complex at temperatures much above ambient.
Moving on from an aromatic monoiodo species, attention then turned to
α
-diiodoperfluoroalkanes where it was possible to demonstrate 2 : 1 com-
plex formation (
14
) [42].
,
ω
Search WWH ::

Custom Search