Chemistry Reference
In-Depth Information
mesogenic behaviour of 4-alkoxybenzoic acids,
5
,dependstotallyonthefact
that they exist as hydrogen-bonded dimers.
More exotic systems have also been described and, for example, Lehn
and co-workers described the complementary system,
6
,whichwasfound
to exhibit a columnar mesophase [18], while neither of the components was
mesomorphic.
Diamides such as
7
and
8
(Fig. 10) were described originally by Mat-
sunaga and Terada [19] and then later reinvestigated by Malthête and co-
workers [20-23]. In many ways, at first sight it is surprising that these systems
are liquid crystalline at all, but the “secret” is in intermolecular hydrogen
bonding,whichcausesthemoleculestoformcolumns.Thesecolumnscan
than organise to form nematic and columnar phases.
However, the hydrogen-bonded mesogens that are of most interest in the
context of this article are those elaborated initially by Kato and Fréchet in
the early 1990s [24-33]. In this approach, a pyridine, which may or may not
have liquid crystal properties, was hydrogen bonded with a 4-substituted ben-
zoic acid to form a new species with its own, distinct mesomorphism. For
example, complex
9
shows a SmA phase that persists to 238
◦
C(
n
=2,
m
=4),
while its free component pyridine is nematic to 213
◦
C; the component ben-
zoic acid is also nematic (as the H-bonded dimer) to 147
◦
C(althoughnote
that the notional “monomer” would not be liquid crystalline).












































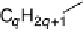





































Search WWH ::

Custom Search