Chemistry Reference
In-Depth Information
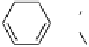
Fig. 5
Schematic representation of the molecular arrangement in
A
main-chain and
B
side-
chain liquid crystal polymers
Many MCLCP are highly insoluble, high-melting materials (if they melt at
all) as they are rigid. However, if they can be processed then they make very
strong materials, an example of which is Kevlar (Fig. 6) - a copolyamide of
terephthalic acid and
p
-phenylene diamine - which is spun into high-strength
fibres from its nematic phase in oleum. One way in which MCLCP can be
made more tractable is if the rigid link between mesogenic groups is replaced
by a flexible chain to give semi-flexible MCLCP.
Fig. 6
Molecular structure of Kevlar®
SCLCP tend to have a conventional backbone, the two most common be-
ing methylsiloxane and (meth)acrylate, the former giving low glass transition
temperatures (
T
g
), while the latter gives high values of
T
g
. In the case of silox-
anes, the mesogenic groups are grafted onto the preformed polymer while for
acrylates, the monomeric unit already contains the mesogenic moiety.
3
Characterisation of Liquid Crystal Mesophases
The principal technique is polarised optical microscopy, which exploits the
anisotropy in refractive index of the liquid crystal mesophases. Thus, the mi-
croscope is configured (Fig. 7) so that the sample is between two polarisers
that are crossed (no light would normally pass through) and the sample is
placed on a heated stage through which light may pass. Plane, polarised light
then impinges on the sample (
<
1 mg of sample held between two micro-






























Search WWH ::

Custom Search