Chemistry Reference
In-Depth Information
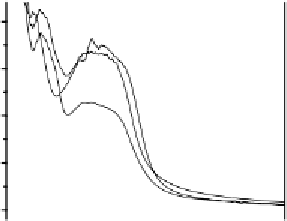
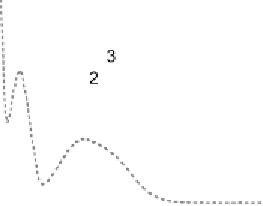
Fig. 9
Solid-state spectra of bromide complexes with TCP (
solid lines
):
1
Pr
4
N
+
[Br
-
,
(TCP)
4
],
2
(Et
4
N
+
)
2
[(Br
-
)
2
,(TCP)
3
],
3
Bu
4
N
+
[Br
-
,(TCP)
4
]. Note: spectra of the corres-
ponding complexes in solution are shown as
gray dashed line
[23]
-acceptors
a
Table 3
Solid-state characteristics of halide associates with
π
X
-
···
C
b
[A]
Molar ratio
Br
-
/TCP
2 : 3
d
3.16
1:4
e
3.15
I
-
/TCP
1 : 2
d
3.52
1:1
f
3.49
c
Cl
-
/TCP
1 : 4
f
3.07
NCS
-
/TCP
1 : 1
f
2.951
i
3.288
j
Br
-
/TCNE
2 : 1
d
3.20
1:1
e
3.11
1:1
f
3.20
I
-
/TCB
c
1:2
g
3.46
1:2
h
3.45
Br
-
/TCB
c
1:2
h
3.34
Br
-
/
o
-CA
1 : 1
e
2.93
a
From [23] unless noted otherwise
b
Halide-carbon distances with closest contacts.
Note that van der Waals radii are (in A) 1.70 (C), 1.52 (O), 1.55 (N), 1.80 (S), 1.80 (Cl),
1.85 (Br), 2.1 (I) [20]
c
From [24]
d
Et
4
N
+
salt
e
Pr
4
N
+
salt
f
Bu
4
N
+
salt
g
Na(18-crown-6)
+
salt
h
K(18-crown-6)
+
salt
i
C
···
Nseparation
j
C
···
Sseparation
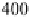
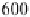



Search WWH ::

Custom Search