Chemistry Reference
In-Depth Information
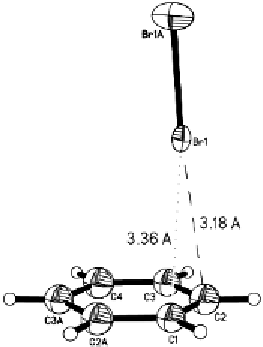
Fig. 6
Molecular structure of the Br
2
complex with benzene (from [68])
calculations, which consistently favor both over-atom and over-bond (i.e.,
η
2
-) coordinations without a significant energy barrier between
them [69-74].
TheonlyreportedX-raystructureofa
1
-and
η
-bonded diiodine exists in the
I
2
/coronene associate [75], which shows the I
2
to be located symmetrically
between the aromatic planes and to form infinite donor/acceptor chains.
η
2
-Coordination of diiodine over the outer ring in this associate is similar to
that observed in the bromine/arene complexes (vide supra), and the I - Csep-
aration of 3.20A is also significantly contracted relative to the sum of their
van der Waals radii [75]. For the highly reactive dichlorine, only X-ray struc-
tures of its associates are observed with the n-type coordination to oxygen of
1,4-dioxane [76], and to the chlorinated fullerene [77].
It is should be noted that high reactivity precludes the X-ray structural
characterization of the
π
-complexes between dihalogens and olefinic accep-
tors. Indeed, quantum mechanical calculation of the interaction between
π
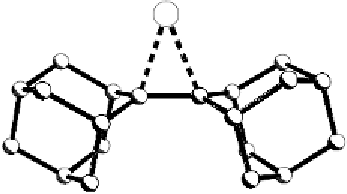
Fig. 7
Molecular structure of adamantylideneadamantane bromonium, as its salt with
Br
5
-
counterion (Rosokha et al. unpublished results)



Search WWH ::

Custom Search