Chemistry Reference
In-Depth Information
dimethano-1,2,3,4,5,6,7,8-octahydroanthracene (DMA) are examples where
distinct bands are observed [53].
No new absorption bands are observed in other cases, largely due to
the fact that the strong absorptions of the aromatic donors obstruct the
UV-spectral measurements. For the complex between CBr
4
and TMPD, the
quantitative analyses of the temperature and concentration-dependent ab-
sorptions of the new band at 380 nm afford the extinction coefficient of
ε
CT
= 3.2
10
3
M
-1
cm
-1
, as well as the thermodynamic parameters for com-
plex formation:
×
S
=- 14 e.u.,and
K
DA
= 0.3 M
-1
at
295 K. Such thermodynamic characteristics are similar to those of the dihalo-
gen complexes of as well as those of other acceptors with aromatic donors.
Similar results are also obtained for CBr
4
associates with halide and thio-
cyanide anions [5, 53].
H
=- 4.5 kcal M
-1
,
∆
∆
2.3
Complexes of Halide Anions with Aromatic and Olefinic π-Acceptors
Although non-covalent interactions of anions are one of the most actively ex-
plored areas of supramolecular chemistry [15], the anion sensing and recog-
nition have up to now relied primarily on electrostatic binding or hydrogen
bonding to the receptor [16, 54-61]. However, recent UV-Vis and NMR spec-
tral studies clearly reveal that complex formation takes place in the solutions
between halides and neutral olefinic and aromatic
π
-acceptors such as those
in Fig. 3 [23, 62].
Fig. 3
π
-acceptors and their identification
SolutionsofthealkylammoniumsaltsofCl
-
,Br
-
,I
-
in acetonitrile show no
visible absorptions beyond 300 nm. The aromatic
-acceptor, tetracyanopy-
razine (TCP) is characterized by strong absorptions in the 220-300 nm range
and a shoulder at 350 nm. However, the electronic spectrum of a mixture of
the bromide salt and TCP reveals a new absorption band at
π
λ
CT
= 400 nm


























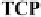



Search WWH ::

Custom Search