Chemistry Reference
In-Depth Information
The term halogen bonding addresses exclusively the former contacts and
its usefulness relies on the identification of a specific subset of the numerous
and diverse non-covalent interactions that halogens can give rise to [58].
TheattractivenatureofXBcausesD
X distances shorter than the sum
of van der Waals radii of involved atoms; the stronger the interaction, the
shorter the D
···
X interaction lengths. Consistent with the rationalization of
XB as an electron donation from D to the antibonding X - Y orbital [59], XB
formation results in an elongation of the X - Ycovalentbond.TheD
···
Xin-
teraction length is usually a more sensitive probe for XB strength than the
X - Ycovalentbondelongation(Table3).
In many cases the XB adduct is a pre-reactive complex (or intermediate)
formed prior to chemical reaction or significant charge transfer [18] (see also
the chapter by Legon in this volume). The stronger interactions easily evolve
into different molecular species if concentration, temperature, solvent polar-
ity, or other parameters are changed [60]. The 1 : 1 complex that dihalogen
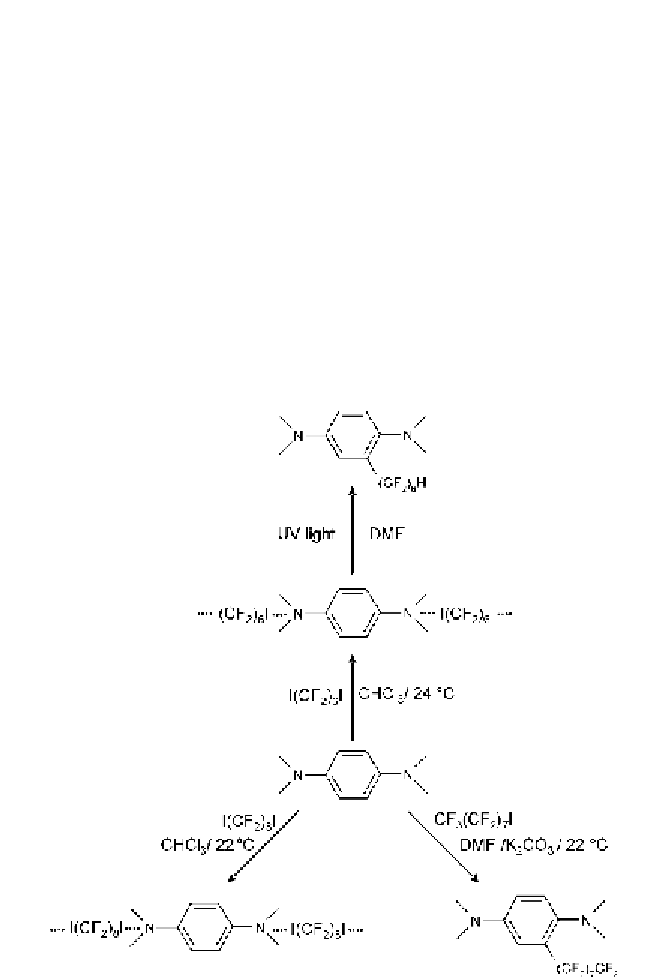
···
Fig. 4
Halogen-bonded adducts are pre-reactive complexes, which, under convenient con-
ditions, can lead to covalent bonds breaking and forming. Perfluoroalkylation occurs
when the complexes between iodoperfluoroalkanes and anilines are heated or irradiated
in certain solvents
Search WWH ::

Custom Search