Chemistry Reference
In-Depth Information
Fig. 1
Schematic representation of XB. XB acceptors (
D
) are neutral or anionic species,
while donors (
X
) are halogen atoms bound to a wide diversity of molecular arrays (
Y
)
Architectures originating from halogens and interhalogens (Fig. 1, Y = F,
Cl, Br, I) gave fundamental contributions towards the identification of the in-
teraction [14-17] and are still attracting great interest [18, 19] (see also the
chapter by Legon in this volume) as it is the case, for instance, for poly-
halide anions [20] or for adducts wherein sulfur, selenium, or arsenic deriva-
tives work as electron donors [21]. Numerous architectures in which the
electrophilic halogen is bound to nitrogen, phosphorous, or other elements
(Fig. 1, Y = N, P, etc.) [22] present the standard XB characteristics (Table 1).
However, the focus of this chapter will be limited to the supramolecular ar-
chitectures formed by carbon-bound halogens (Fig. 1, Y = C).
Both n and
electrons can be involved in XB formation [23-27] and usu-
ally the latter give weaker interactions than the former [18, 28] (see also the
chapter by Legon and the chapter by Kochi in this volume). In this chapter we
will consider only n XB acceptors.
The first unequivocal report on a halogen-bonded complex was made by
F. Guthrie who described, in 1863, a solution-based synthesis of the NH
3
···
π
I
2
complex [29]. In 1881 J. W. Mallet described an alternative synthesis for
the same compound based on a gas/solid protocol [30]. Two years later
O. Roussopoulos described the 1 : 3 co-crystal formation between iodoform
and quinoline as well as other related adducts [31, 32]. In 1893 I. Remsen
and J. F. Norris demonstrated the tendency of methylamines to form similar
adducts with chlorine, bromine, and iodine [33]. It can thus be stated that the
basics in the topic of halogen-bonded adducts had been laid in the nineteenth
century. The field begun to be systematized only after the crystallographic
studies of O. Hassel in the 1950s. In his Nobel lecture, Hassel stressed the sim-
ilarities between interactions wherein hydrogen and halogen work as electron
acceptors, and also the similarities between interactions given by dihalogens
and halocarbons [17]. Important contributions to the topic and its systemati-
zation have been given by H. A. Bent [16] and J.-M. Dumas [34].
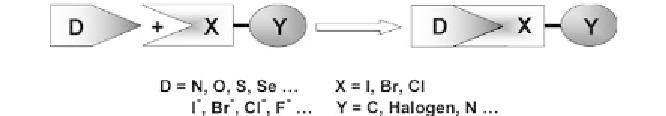
Search WWH ::

Custom Search