Environmental Engineering Reference
In-Depth Information
Water-Soluble Ions
With ion chromatography (IC) four water-soluble anions (i) and five water-soluble
cations (ii) could be detected:
1. Sulphate, nitrate, chloride and luoride
2. Sodium, ammonium, potassium, magnesium and calcium
Sulphate (SO
4
2−
) was the prevalent anion with an average concentration of 16.2 ±
10.2 µg/m
3
for PM
2.5
and 33.9 ± 14.7 µg/m
3
for TSP samples in 2006 (all mean
values in this paper are named plus/minus standard deviation). The mean sulphate
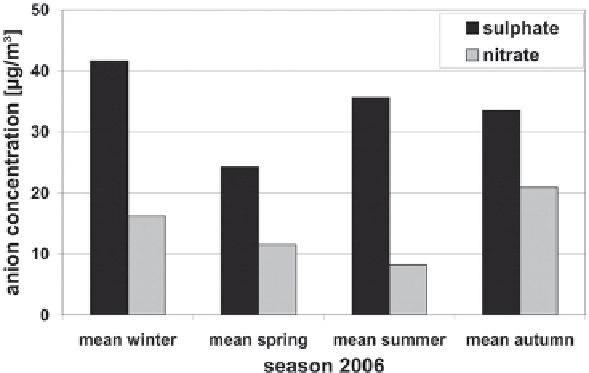
concentrations for the different seasons are displayed in Fig.
6
for TSP samples
from site 4. The highest sulphate concentrations occurred in winter with an average
concentration 41.6 ± 17.4 µg/m
3
and a maximum weekly value 70.4 µg/m
3
. Summer
values are also quite high with 35.6 ± 12.5 µg/m
3
, on average. In summer, a strong
secondary formation of sulphate, caused by the strong solar radiation and high
humidity, is assumable. In winter, the burning of fossil fuels, and especially of coal,
probably contributes considerably to the high sulphate concentrations.
Nitrate, the second most abundant water-soluble anion, showed a different seasonal
trend. Lowest nitrate concentrations were reached in summer time (8.2 ± 4.5 µg/m
3
for
TSP), whereas winter and spring concentrations were high (winter: 16.2 ± 7.5 µg/m
3
,
spring: 11.5 ± 5.9 µg/m
3
for TSP). The seasonal mean concentrations of TSP samples
(site 4) are plotted in Fig.
6
. The annual course for nitrate concentrations in TSP
samples was similar to the course of total mass concentration (r = 0.72, n = 53).
Sulphate mainly originates from stationary sources (industrial or household burning
of fossil fuels/coal), whereas the main source for nitrate are mobile sources (especially
traffic emissions)
[7, 8]
). In this regard, the sulphate/nitrate ratio is crucial (Fig.
7
).
Fig. 6
Concentration of water-soluble sulphate and nitrate from TSP samples at site 4 for the
different seasons in 2006