Environmental Engineering Reference
In-Depth Information
Fe
2+
+ 1/4O
2
+ 3/2H
2
O → FeOOH + 2H
+
Fe
3+
+ 2H
2
O → FeOOH + 3H
+
(1)
Al
3+
+ 3H2O → Al(OH)
3
+ 3 H
+
Mn
2+
+ 1/4O
2
+ 3/2H
2
O → MnOOH + 2H
+
These reactions are used to calculate the acidity produced by ARD using the
expression: Acidity = 50[2Fe
2+
/56 + 3Fe
3+
/56 + 3Al
3+
/27 + 2Mn
2+
/55 + 1,000(10
−pH
)],
where metal concentrations are in mg/L and 50 is the equivalent weight of CaCO
3
[8]
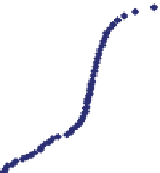
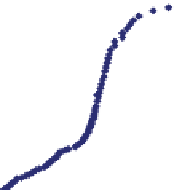
. Water samples were taken in winter 2008 for analysis at S1 and an acidity curve
was performed at the S1 station. This curve, in addition with another one corre-
sponding to the winter 2006, is shown in Fig.
4
. The main properties of the water
at the S1 sampling point (La Silva) were: 2.75 pH, 56 mg/L Fe
2+
, 108 mg/L Fe
3+
,
504 mg/L Al
3+
, 8.96 mg/L Mn
2+
. Applying the above expression, the acidity was
calculated to 3,289 mg/L CaCO
3
. As can be seen in Fig.
4
, the total acidity is close
to the value obtained by the expression
[8]
.
The acidity at S1 is very high (3,289 mg/L CaCO
3
), and the alkalinity of the
tributaries is very low. Thus they are not capable to neutralize the acidity, except for
3000
2500
2000
1500
1000
500
0
2
3
4
5
6
7
8
9
10
3000
2500
2000
1500
1000
500
0
2
3
4
5
6
7
8
9
10
Fig. 4
Acidity curves in La Silva headwaters (acidity: units in mg/L CaCO
3
)