Geology Reference
In-Depth Information
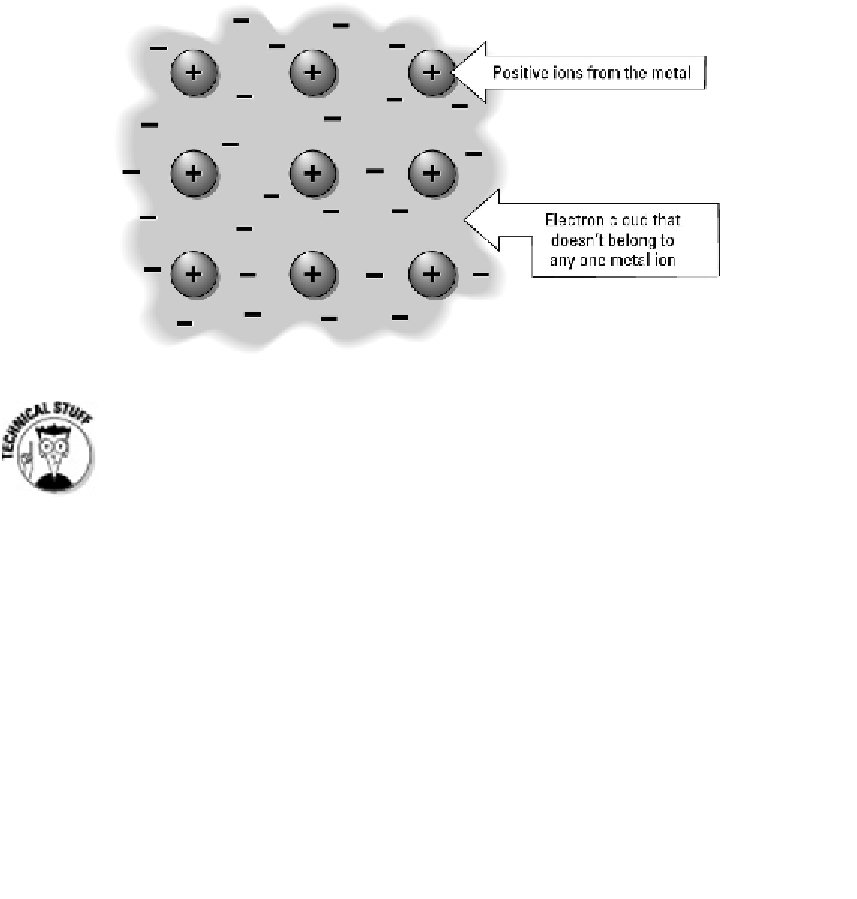
The unique nature of metallic bonds is what gives metals such as gold or sil-
ver their unique characteristics. The ability to conduct electrical current is a res-
ult of the movement of electrons. The shiny appearance is due to the large num-
ber of freely floating electrons. And the fact that metals can be bent and molded
without breaking is also a result of the movement of electrons between atoms in
the metallic bond.
Formulating Compounds
The bonding of elements to form compounds is fundamental to understanding the form-
ation of rocks and minerals (which I describe in Chapter 6). When scientists discuss the
processes of rock formation, as well as other earth processes involving chemical
changes (such as weathering, described in Chapter 7), they use a shorthand of chemical
formulas.
The chemical formula of a compound describes the number of different atoms
of each element that are combined into a compound. For example, the chemical
formula for quartz is as follows: