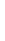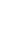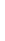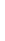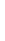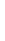Geology Reference
In-Depth Information
Sodium
Na
2.8
Potassium
K
2.6
Magnesium Mg
2.1
Interpreting isotopes
Most elements exist as atoms of different atomic mass number, indicating different num-
bers of neutrons in the nucleus. As long as the number of protons stays the same (the
atomic number), you have the same element, but its atomic mass changes with the addi-
tion or subtraction of neutrons. These various atoms of the same element with different
atomic mass numbers are called
isotopes.
Take, for example, the element carbon, which has three common isotopes:
Carbon-12 has six protons and six neutrons.
Carbon-13 has six protons and seven neutrons.
Carbon-14 also has six protons but has eight neutrons.
Isotopes are very useful because while the element is the same (such as Car-
bon-12, Carbon-13, and Carbon-14), the heavier isotope reacts differently in chem-
ical reactions. This means the isotopes can be counted or measured to interpret
conditions of temperature or pressure when a chemical reaction occurred in the
past. Also, some isotopes change or decay over time at a measurable and con-
stant rate, which makes them useful for measuring time. You find details about
how isotopes are used to determine the age of rocks in later chapters.
Charging particles: Ions
Each subatomic particle in an atom has a
charge,
similar to the way opposite ends of a
battery or magnet are charged: positive or negative. In an atom, the protons are posit-
ive, the neutrons are neutral (no charge), and the electrons are negative. Most atoms
have the same number of protons and electrons, which means the atom itself has no
charge; it's neutral.