Geology Reference
In-Depth Information
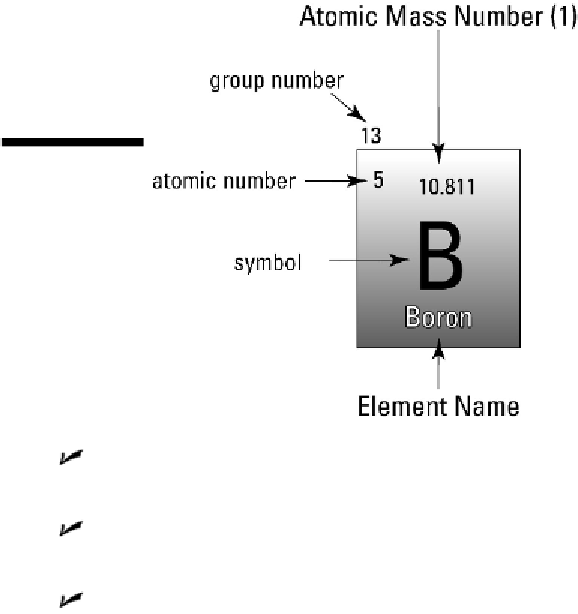
Figure 5-2:
The
parts of one
square of the peri-
odic table of ele-
ments.
Atomic mass number:
The
atomic mass number
of an element is the total number
of protons and neutrons in its nucleus.
Atomic number:
The
atomic number
of an element is the number of protons in its
nucleus.
Group number:
The
groupnumber
tells you how many electrons in the atom are
located in the outermost orbital shell and are, therefore, available to bond it to
other atoms. For example, elements in Group I have one electron in the outer elec-
tron shell, and Group II elements have two electrons in the outer electron shell.
The group number for each element may help you understand why some ele-
ments, such as Magnesium (Mg) and Calcium (Ca), which are both in Group II, re-
act in similar ways during rock formation and other geologic processes.
Symbol:
The letters on the periodic table are the symbols for each element. These
symbols are a shorthand so that when combinations of elements or chemical reac-
tions are described you don't have to write each element's entire name. The sym-
bols of the periodic table are the same all over the world to make it easier for sci-
entists to communicate.
In many cases, the elemental symbol is based on the name of an ele-
ment in a different language and may not make sense in your native language. For
example, the symbol for gold is Au because in Latin the word for gold is
Aurum,