Geology Reference
In-Depth Information
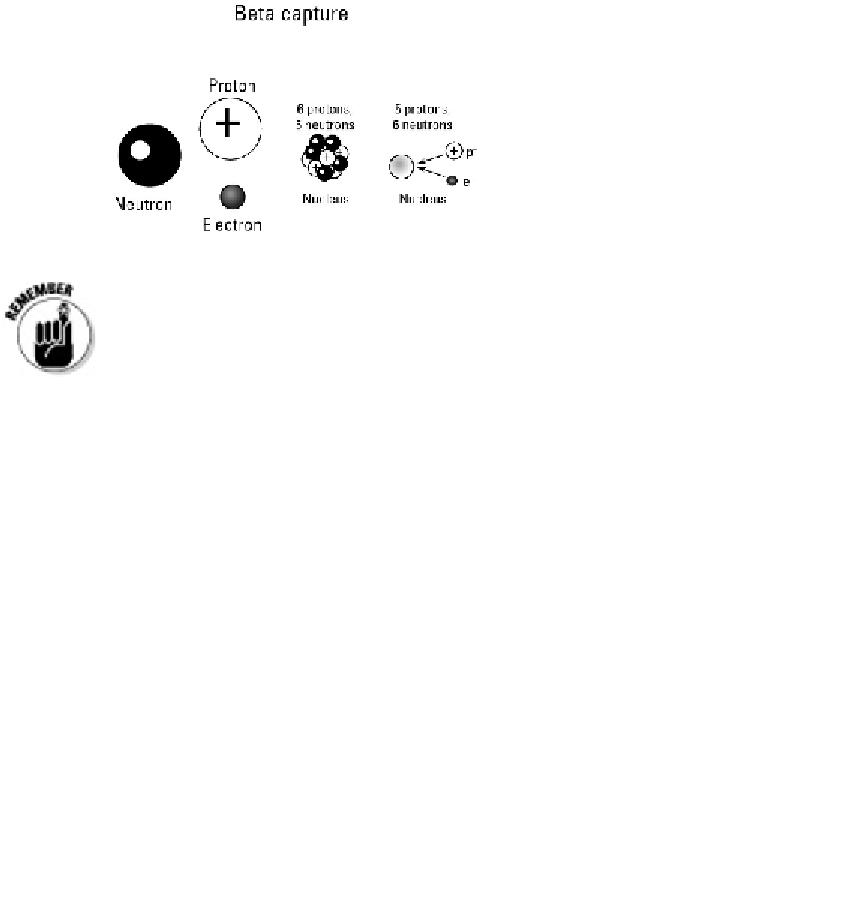
Keep in mind that not all elements have isotopes, and not all isotopes are ra-
dioactive. Only unstable isotopes are radioactive, and after they transform into
stable isotopes of another element, they are no longer radioactive.
Fortunately for scientists, the transformations of radioactive isotopes occur in such a
way that they can be measured and used to date the formation of minerals.
It takes a half-life: Transforming parent isotopes to daughter isotopes
Radioactive isotopes begin as one element and, through decay, become another. The ori-
ginal element is called the
parent isotope,
and the resulting element is called the
daughter
isotope.
Comparing the number of parent and daughter isotopes and knowing how long
it takes for each radioactive element to decay is what provides a numerical age for
rocks.
When a rock forms from cooling magma (see Chapter 7), minerals are formed that incor-
porate into their crystal structure whichever elements are available. (I explain minerals'
crystal structure in Chapter 5.) For example, a mineral of biotite will use potassium
atoms in its crystals. If some radioactive isotopes of potassium are in the magma, then
some of the potassium atoms that form biotite minerals from that magma will be radio-
active. Over time, the unstable potassium isotopes in the biotite mineral decay, trans-
forming into argon atoms, but they remain part of the biotite mineral structure.
The decay of a radioactive isotope can be measured in half-lives. The
half-life
is the amount of time it takes for half the parent isotope atoms to decay into
daughter isotope atoms.