Biology Reference
In-Depth Information
fibrils
that show red-green bi-refringence after CR-staining and a fibrillar appear-
ance in EM. In addition to these criteria, it has been suggested that the term
“amyloid” should be reserved for extracellular aggregates. Interestingly,
fibrils indistinguishable from
ex vivo
fibrils can be formed upon incubation
of the corresponding synthetic peptide. For simplicity, “amyloid” will in this
chapter be used also for intracellular fibrils and synthetic fibrils (Fig. 2).
Today, around 30 human amyloidoses have been described, each
associated with a specific protein. These diseases can be systemic or organ
specific, and are often associated with an increased production of the
deposited protein or the expression of a more amyloidogenic variant of the
protein. The different amyloidoses include a wide variety of proteins and
peptides, and it is not obvious why they polymerize into amyloid. It is
possible that most proteins could form amyloid under certain conditions,
indicating that the polypeptide backbone has an inherited propensity to
Thus, amyloid is composed of proteins arranged in ordered cross-
β
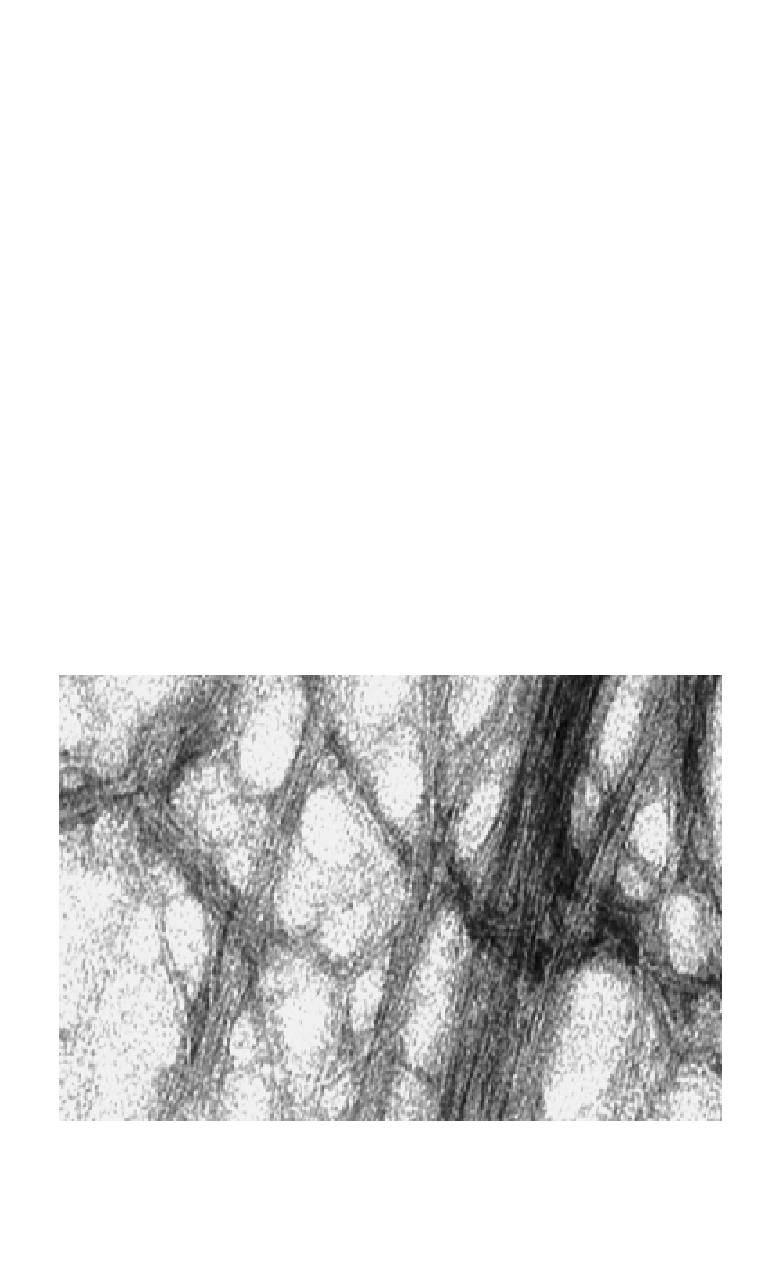
Fig. 2.
Amyloid fibrils formed by Aβ1-40. Synthetic Aβ1-40 was incubated in buffer,
pH 7.4, for three days. After a brief centrifugation, 8 µ l of the sample was placed on an
EM grid and stained with uranyl acetate and examined by EM. Scale bar = 100 nm. (EM
by Johan Thyberg, Karolinska Institutet.)


Search WWH ::

Custom Search