Biology Reference
In-Depth Information
heat transfer, i.e. the temperature difference, is largest at the beginning,
and declines towards the end of the crystallization. This leads to the tem-
perature falling quickly at the beginning, and slower towards the end. The
normal thing therefore is for a massive, uncontrolled primary nucleation
to occur early, resulting in a small product size. Large variations will be
observed from one batch to another. If large crystals are desired, a careful
generation of supersaturation is called for, in particular at the beginning of
the process. As the crystal surface area consuming the supersaturation
increases, it is possible to generate supersaturation faster. This procedure
is called
controlled cooling
(Mullin, Nyvlt, 1971) and the main principle
is that careful cooling should be employed initially, followed by a gradual
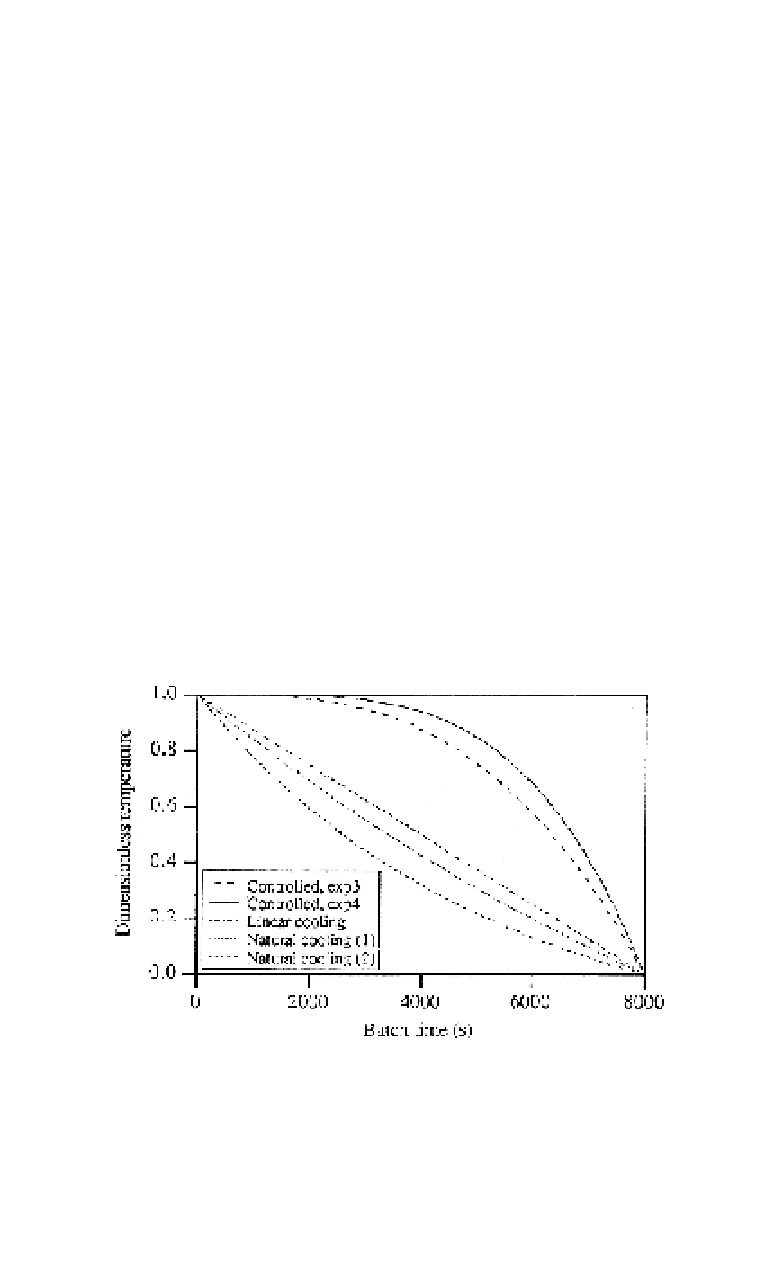
increase in cooling rate as the total crystal surface area increases. In Fig. 8,
a few different controlled cooling profiles are shown, together with two
examples of natural cooling profiles.
In the case of natural cooling, supersaturation peaks very high and
marked, leading to a powerful primary nucleation early on in the process.
Fig. 8.
Different cooling profiles in batch cooling crystallization. The diagram shows the
temperature of the solution as (
T
0
−
T
f
) versus time when the batch is cooled from
the initial temperature,
T
0
, to the final temperature,
T
f
.
T
is the current temperature. In con-
trolled cooling, different exponents,
T
)/(
T
0
−
α,
can be used in the equation describing the cooling
profile: (
T
0
−
(
t
/
t
f
)
α
, where
t
f
is the total cooling time. Natural cooling is
described in the text and is given in the figure for two different final temperatures at equal
cooling water temperature.
T
)/(
T
0
−
T
f
)
=


Search WWH ::

Custom Search