Biology Reference
In-Depth Information
Solution adhering to the surface
(blue line with red dots around crystal)
Incorporation into the lattice
(red dots inside crystal)
Macroscopic cavities inside the
crystal
(blue cavity with red dots inside crystal)
Adsorbed in lattice channels
and cavities
(not shown)
Red dots denote impurity
Blue denotes solution
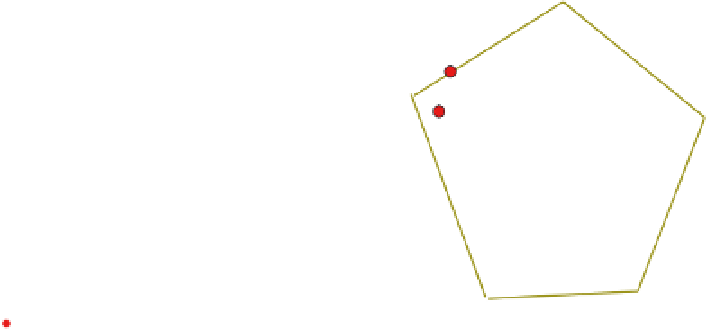
Fig. 7.
Modes of impurity incorporation into a crystalline material. After separation of
crystals from the solution, there is solution with impurities that remains on the surface of
the crystal. Because of poor molecular separation, impurities can be integrated into the lat-
tice. Under poor crystal growth conditions, cavities can be formed trapping impure mother
solution inside the crystal. Sometimes the crystal structure contains channels and cavities
in which impurities can be trapped.
damaged edges and corners are regenerated. A change in the design of the
stirrer can reduce the mechanical damage the crystals are subjected to (de
Jong 1984). Inclusion formation can to some extent be prevented by the
presence of ionic impurities, change of solvent, increased viscosity or the
usage of ultrasound.
The possibility of easily washing away the process solution adhering
to the surfaces of the crystals is strongly linked to the filterability of the
product. If it is hard to filter the crystals, it will be hard to wash them. As
mentioned earlier, the filterability mainly depends on particle size and
shape. Larger, more spherical crystals improve the possibility of washing
off process solution adhering to the surface.
For some processes, agglomeration is desirable since the product par-
ticles become larger and thus easier to filter. However, the agglomerates
may contain process solution, which leads to an impure product, and occlu-
sions may be difficult to wash away. Occlusion problems must primarily
























Search WWH ::

Custom Search