Agriculture Reference
In-Depth Information
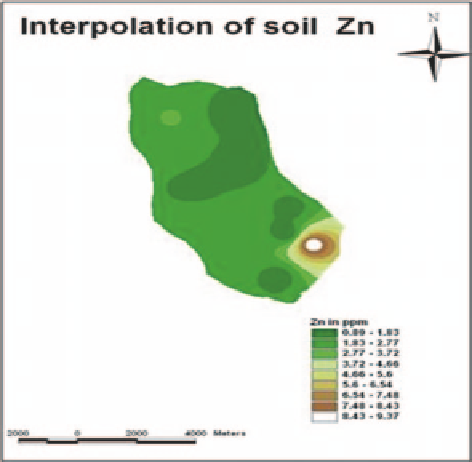
Fig. 4.7
Interpolation of
soil Zn
chlorosis, small sickle-shape leaves, clustered at the tips of shoots, etc. However,
Tolhurst (
1961
) reported that deficiency is prominent in low-country. Tolhurst's ob-
servation can be justified with Hasselo's (
1965
) one, according to which Zn concen-
tration in upcountry flush is almost double compared to low-grown tea.
Though Zn level in groundwater is insignificant, its concentration in soil is sig-
nificant. According to Hettiarachchi and Gupta (
2008
) and Tolhurst (
1961
), Zn is
identified as promising, yield-responding nutrient. Therefore, application of Zn as a
foliar spray was recommended. As seen from the analysis, in most part of the study
area concentration of Zn is well above 1 ppm and it is quite sufficient for plant
growth (Fig.
4.7
).
Lead concentration in ground water is ranging from 0.07 to 0.11 ppm. However,
maximum permissible limit for lead in drinking water is 0.05 ppm. Higher level of
lead in groundwater might be due to intense gem mining activity in the area. How-
ever, further investigations are required for a proper conclusion.
In most part of the study area, manganese concentration in groundwater exceeds
the permissible limit of 0.05 ppm. Soil and groundwater manganese concentrations
are ranging from 0.01 to 0.3 ppm and 0.66 to 2.78 ppm respectively. Availability of
manganese is directly related to soil pH and aeration. In highly alkaline soils, Mn
deficiency is common and in highly acidic soils its concentration goes high turning
toxic for plants. Considering the tea plant nutrient, symptoms of Manganese toxic-
ity were reported from low-country frequently during the dry weather condition
(Hettiarachchi and Gupta
2008
). Furthermore, the toxicity is characterized by the
leaves chlorosis with pronounced green network of the veins. According to Tolhurst
(
1973
), higher level of N application increases the content of Mn in leaves. Accord-