Biomedical Engineering Reference
In-Depth Information
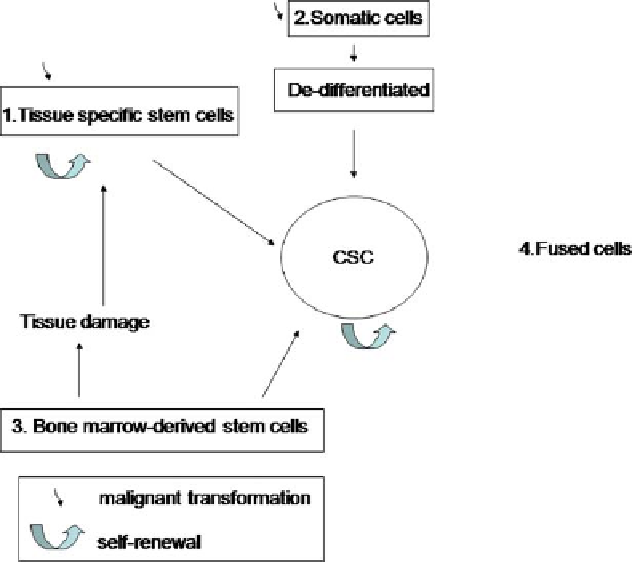
Fig. 2 Normal tissue stem cells are defined by three common proprieties: first, the presence of
an extensive capacity for self-renewal that allows maintenance of the undifferentiated stem
cell pool; second, strict regulation of stem cell number: the asymmetric division into another
stem cell and one progenitor cell that further differentiates into the mature progeny compris-
ing the adult tissue. Third the capacity to differentiate into particular mature cell types.
Therefore, it is possible that a minor subversion of normal stem cell might be sufficient to
create a malignant condition (1). Like normal stem cells, CSCs can self-renew and give rise to
heterogeneous population of daughter cells and proliferate extensively. On the other hand,
mutation in somatic cells might re-program these to CSCs (2). Bone-marrow-derived CD34+
stem cells can migrate to the site of tissue damage where they become tissue-specific stem cells
and are prone to malignant transformation (4). Finally, stem cell can fuse with somatic cells
and in this way found a cancer stem cell. The transforming event could occur in the same stem
cell, the somatic cells or the fused cell. Self-renewal is indicated by a curved arrow
(i.e. E-cadherin, P-cadherin and desmoglein), upregulation of receptors and
signalling molecules important for melanoma cellmelanoma cell and melanoma
cell-fibroblast interactions (i.e. N-cadherin, zanula occludens protein -1) and
deregulation of morphogenesis such as Notch receptors and their ligands. The
investigation of normal melanocyte homeostasis might help us to define how
melanoma and, in particular, melanoma cancer stem cells escape the micro-
environment created by epidermal keratinocytes and how they develop new
cellular partners in fibroblasts and endothelial cells which support their growth
and invasion (Haas and Herlyn, 2005).