Biomedical Engineering Reference
In-Depth Information
demonstrated many years ago by Potter Laboratory that is organized into
proliferative units (Allen and Potten, 1974; Potten, 1974). Accumulated evi-
dences indicate that keratinocyte stem cells (KSCs) reside in the bulge area of
the hair follicle both in rodent and human (Cotsarelis et al., 1990; Lyle et al.,
1998; Morris and Potten, 1999; Taylor et al., 2000). However, there are also
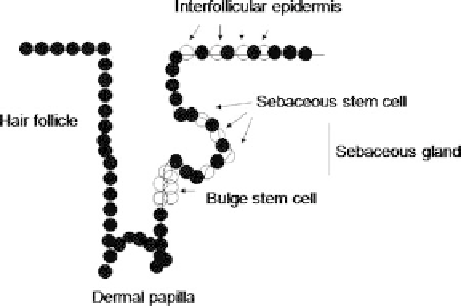
stem cells in the interfollicular epidermis and, potentially, in the sebaceous
gland (Fig. 1). The stem cell progeny that are destined to terminally differentiate
can first undergo a few rounds of divisions, during which time they are known
as transit-amplifying cells (Niemann and Watt, 2002). Thereby, at present it is
unclear whether transit-amplifying cells have multilineage differentiation
potential or they are lineage-restricted.
Fig. 1 The epidermis is a multilayered epithelium that covers the skin providing a waterproof
barrier that essentially controls the rate of water loss from the body. The different types of
cells play an important role in maintaining the normal functions in the skin. In this figure is
shown the localization of stem cells in mammalian epidermis. The terminally differentiated
cells in all regions of the epidermis are continually shed from the skin and must be replaced
throughout adult life. The replacement depends on the stem cells. These cells show an
extensive self-renewal capacity and produce progeny that undergo terminal differentiation
along the different epidermal lineages. Stem cells are present at the follicle bulge, at the basal
layer of the interfollicular epidermis. There are conflicting reports as to whether they are
clustered (like as in the hair follicle) or distributed singly. There are a third stem cell
population in the sebaceous gland. However, it is possible that the latter is maintained by
bulge stem cells. Furthermore, there are transit-amplifying cells and cells that are withdrawn
from the cell cycle and become committed to terminal differentiation
Molecular signature of living bulge cells was delineated and their biological
behaviour in vitro and in vivo was studied (Ohyama et al., 2006; Tumbar et al.,
2004; Morris et al., 2004; Blanpain et al., 2004). In particular, the molecule
signatures of the mouse bulge cells were successfully obtained by two groups
(Tumbar et al., 2004; Morris et al., 2004), identifying 57 overlapping genes
upregulated including genes associated with growth arrest or proliferation and