Environmental Engineering Reference
In-Depth Information
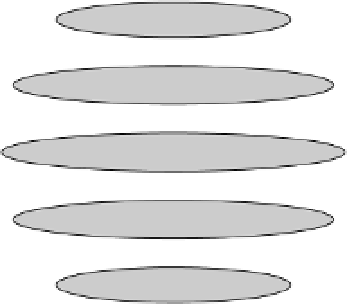
Figure 3.12 Five allowed orientations of angular momentum l ¼2, length of vector and z
projections in units of
. Azimuthal angle
j
is free to take any value.
h
m ¼l
; l þ
1
;
...
; ðl
1
Þ;
l
:
ð
3
:
29
Þ
There are 2
l þ
1 possibilities. Again,
m
represents the projection of the angular
momentumvector along the
z
-axis, in units of
h
.For
l ¼
1, for example, there are three
values of
m
,
1, 0, and 1, and this is referred to as a triplet state. In this situation, the
angular momentum vector has three distinct orientations with respect to the
z
-axis:
polar angle
¼
45
,90
, and 135
. In this common notation, the
n¼
2 state
(containing four distinct sets of quantum numbers) separates into a singlet (2s)
and a triplet (2p).
For each electron, there is also a spin angular momentum vector
S
with length (
s
(
s þ
1))
1/2
h
, where
s ¼
1/2, and projection
m
s
¼ð
1
=
2
Þ
h
:
ð
3
:
30
Þ
These strange rules, mathematically required to solve Schrodingers equation,
are known to accurately describe the behavior of electrons in atoms. We can use
these rules to enumerate the possible distinct quantum states for a given energy
state,
n
.
Following these rules, one can see that the number of distinct quantum states for a
given
n
is 2
n
2
(Figure 3.12). Since the Pauli exclusion principle for electrons (and
other Fermi particles) allows only one electron in each distinct quantum state, 2
n
2
is
also the number of electrons that can be accommodated in the
n
th electron shell of an
atom. For
n¼
3 this gives 18, which is seen to be twice the number of entries in
Table 3.1 for
n¼
3. The factor of two represents the spin degeneracy.
Another peculiarity of angular momentum is that the vector
p
(
l
L
has length
L¼
(
l þ
1))
h
and projection
L
z
¼m
h
. A similar situation occurs for the spin vector
S
,with
p
(
s
(
s þ
1))
h
and projection
m
s
h
. For a single electron
m
s
¼
1/2.
magnitude
S¼