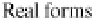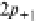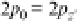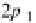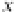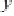Environmental Engineering Reference
In-Depth Information
complex, and therefore the electron in this state has no orbital angular momentum.
Both of these features correct errors of the Bohr model.
The
n¼
2 wavefunctions start with
Y
210
, which exhibits a node in
r
, but is
spherically symmetric. The
first anisotropic (nonspherical) wavefunctions are
Y
21
;
1
¼ RðrÞf ðyÞgðjÞ¼C
2
r
sin
y
e
r=
2
exp
ðijÞ;
ð
3
:
26
Þ
where
r¼Zr
/
a
o
.
These are the
first two wavefunctions to exhibit orbital angular momentum, here
h
along the
z
-axis. Generally,
gðjÞ¼
exp
ðim
jÞ;
ð
3
:
27
Þ
where
m
, known as the magnetic quantum number, represents the projection of the
orbital angular momentum vector of the electron along the
z
-direction, in units of
h
.
The orbital angularmomentum
L
of the electronmotion is described by the quantum
numbers
l
and
m
.
The orbital angular momentum quantum number
l
has a restricted range of
integer values:
l ¼
0
;
;
...
;
n
1
:
ð
3
:
28
Þ
1
2
This rule con
rms that the ground state,
n¼
1, has zero angular momentum. In
the literature the letters s, p, d, f, and g, respectively, are often used to indicate
l ¼
0, 1,
2, 3, and 4. So a 2s wavefunction has
n¼
2 and
l ¼
0, and the wavefunctions sketched
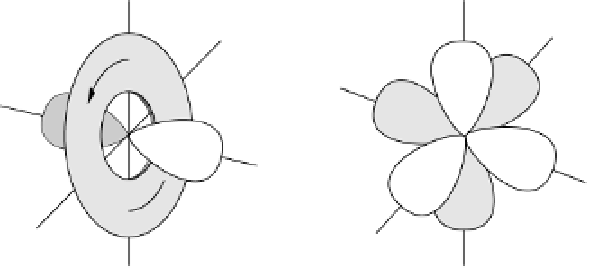
in Figure 3.11 are called the 2p wavefunctions.
The allowed values of the
magnetic quantum number m
depend upon both
n
and
l
according to the scheme
-
-
Figure 3.11 2p (n¼2, l ¼1) wavefunctions in schematic form. Left panel, complex forms carry
angular momentum. Right panel, linear combinations having the same energy now assume aspect
of bonds.