Environmental Engineering Reference
In-Depth Information
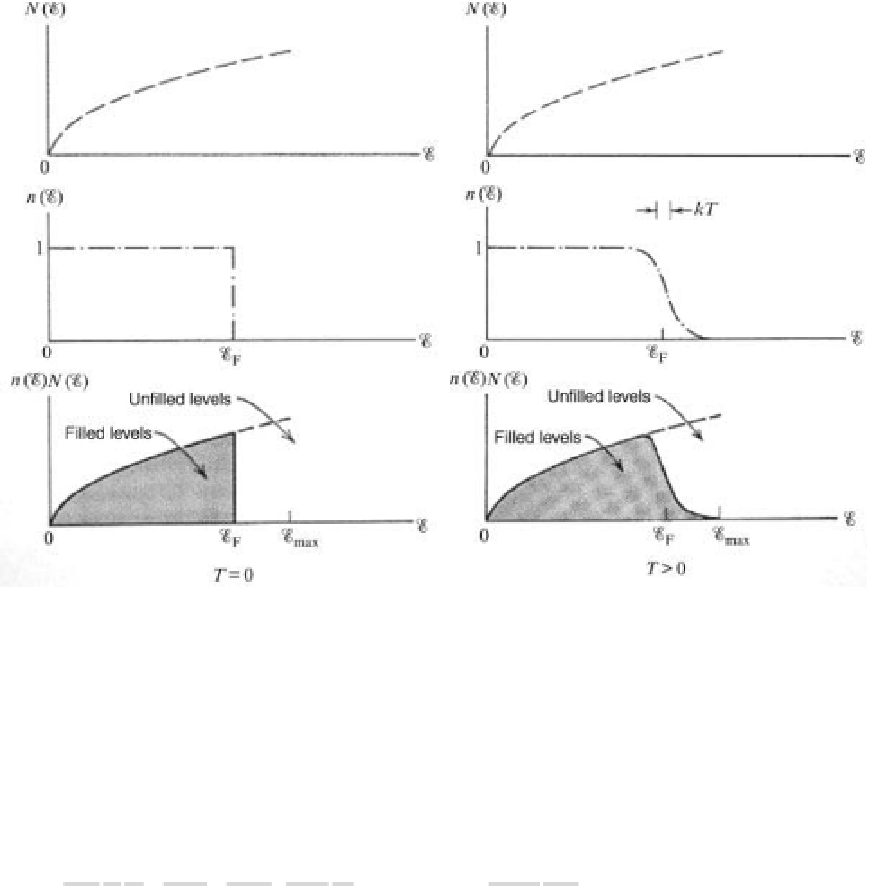
Figure 3.10 Density of states g(E) and occupation f(E)atT¼0 (left) and T nonzero (right) in 3D
case.
Equation 2.9 is easily extended to 3D
x
-,
y
-,
z
-coordinates (see Equation 2.16a in
connection with solutions in the box of side
L
) but is more complicated when
expressed in spherical polar coordinates (2.19). Using these coordinates, for a
spherically symmetric potential
U
(
r
), one
nds, where
and
j
, respectively, are the
polar and azimuthal angles:
h
2
2
2
m
r
2
2
2
mr
2
h
r
2
q
1
q
y
q
1
sin
q
q
y
y
q
y
q
y
1
sin
2
q
y
q
j
y
¼ E
y
sin
þ
þUðrÞ
:
r
r
y
2
q
y
ð
3
:
21
Þ
The Schrodinger equation is applied to the hydrogen atom, and any one-electron
atom with nuclear charge
Z
, by choosing
U¼k
C
Ze
2
/
r
, where
k
C
is the Coulomb
constant. It is found, because of the spherical symmetry, that the equation separates
into three equations, in variables
r
,
, and
j
, by setting
y ¼ RðrÞf ðyÞgðjÞ:
ð
3
:
22
Þ
The solutions are conventionally described as the quantum states
Y
n
,
l
,
m
, speci
ed
by quantum numbers
n
,
l
,
m
.
The principal quantum number
n
, setting the energy, is associated with the
solutions for the radial wavefunction,