Environmental Engineering Reference
In-Depth Information
J
10
19
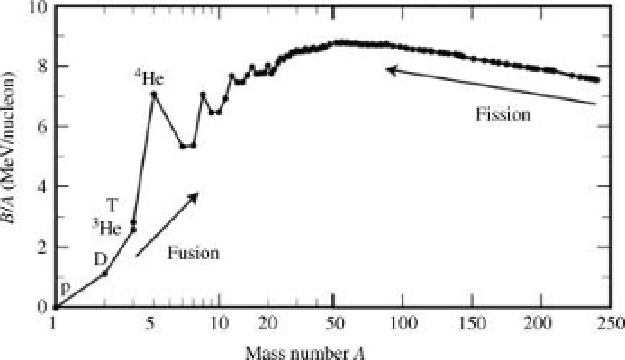
Ws. Chemical reactions release energy on the order of 1 eV per atom,
while nuclear reactions release energies on the order of 1MeV per atom, see
Figure 1.5. A broad distribution of particle speed v is allowed in the normalized
Maxwell
-
Boltzmann speed distribution,
¼
1.6
3
=
2
4
p
v
2
exp
mv
2
D
ð
v
Þ¼ð
m
=
2
p
k
B
T
Þ
ð
=
2 k
B
T
Þ:
ð
1
:
9
Þ
While one may have learned of this in connection with the speeds of oxygen
molecules in air, it usefully applies to the motions of protons at 15 million K in the
core of the sun.
The most probable speed is (2 kT/m)
1/2
that corresponds to a kinetic energy E
k
¼
1/2 mv
2
of kT. In connection with the probability of tunneling through the Coulomb
barrier, which rises rapidly with rising interparticle energy (particle speed), one sees
that the high-speed tail of the Maxwell
-
Boltzmann speed distribution is important.
The overlap of the speed distribution, falling with energy, and the tunneling
probability, rising with energy, typically as exp[
(E
G
/E
k
)
1/2
] as we will learn later,
leads to what is known as the Gamow peak for fusion reactions in the sun. (The
suns neutrino output has been measured on earth, and is now regarded as in
satisfactory agreement with the p
-
p reaction rate in the core of the sun [9].)
The energy release of this reaction can be calculated from the change in the m
i
c
2
terms. Using atomic mass units u,wegofrom4
1.0078 to 4.0026
þ
2(1/1836)
¼
nd 8.89MeV per
4
He, neglecting the
neutrino energy. The atomic mass unit u is nearly the protonmass, but de
ned in fact
as 1/12 the mass of the carbon 12 nucleus.
We should point out the large scale of the fusion energy release, here nearly 9MeV
on a single atom basis. This is about a million times larger than a typical chemical
reaction, on a single molecule basis. The nuclear force that binds the protons and
neutrons in the nuclei is indeed about a million times stronger than the typical
10
3
u, and using 935.1MeV as uc
2
,we
9.51
Figure 1.5 The sun
s radiating power comes largely from nuclear fusion of protons p into
4
He at
15million K. Mass (nucleon) number A¼Z þ N. p, D, and T, are equivalent, respectively, to
1
H,
2
H,
and
3
H. (reproduced from Ref. [8], Figure 1).