Environmental Engineering Reference
In-Depth Information
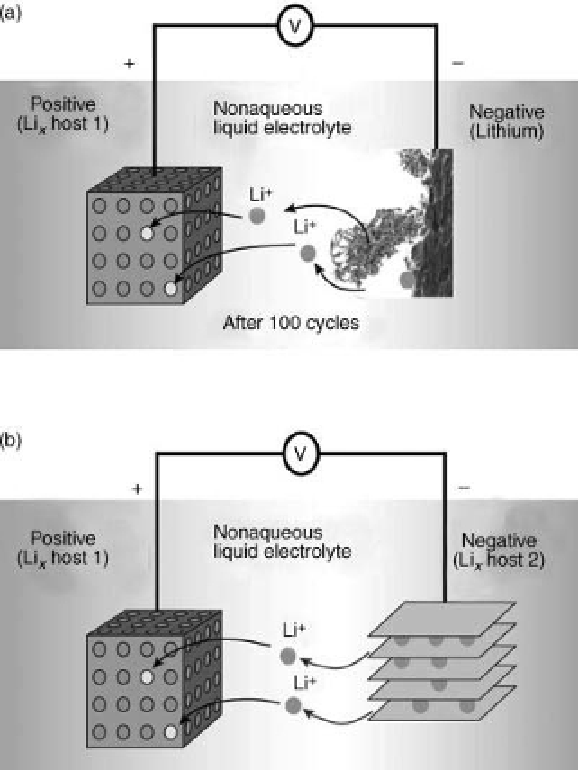
Figure 10.9 Operating principle [142] of
rechargeable Li-ion battery. Li ions diffuse from
high-energy sites in the graphite anode to low-
energy sites in the cathode, driving charge
around the external circuit. Cells using
Li
1x
CoO
2
(left) and Li
x
intercalated into
graphite (right) provide 3.6 V, energy densities
120
-
150Wh/kg are widely used in portable
electronic devices. Li ions reversibly enter
(intercalate) and leave weakly bound positions
between the graphene carbon layers of graphite.
abundance of iron, and freedom from overheating. Another advantage is that in the
chemical reaction in the LFP case, relative to that shown in Equation 10.3, the value of
x
goes completely to zero, leaving no residual Li in the cathode when fully charged.
The chemical reaction for one form of Li-ion cell is
Li
1
x
CoO
2
þ
Li
x
C
6
¼
C
6
þ
LiCoO
2
;
ð
10
:
3
Þ
where the carbon is a graphite electrode.
Ions, not electrons, are the current carriers
. Note
that lithium ions are not oxidized. In a lithium ion battery, the positive lithium ions
flow internally from the graphite anode to the cathode, with the transition metal,
cobalt, in Li
1
x
CoO
2
being reduced from Co
4
þ
to Co
3
þ
during discharge. The
performance may be as high as 160Wh/kg.