Environmental Engineering Reference
In-Depth Information
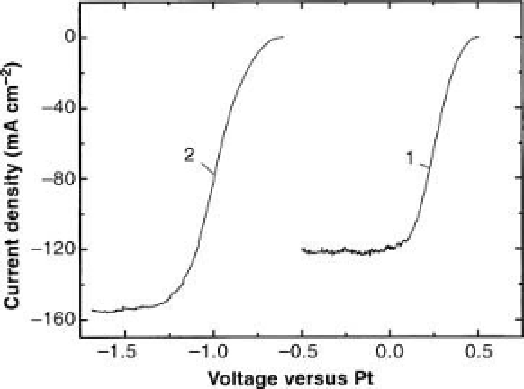
Figure 9.3 Current
voltage diagrams [111] of
tandem cell (right, curve 1) and single right-
hand cell (p-GaInP
2
cell) under white light
illumination. A voltage source has been inserted
in the upper connecting wire as shown in
Figure 9.2, to obtain curve 1. The voltage source
has been inserted between the platinum
electrode and the inner ohmic junction (labeled
transparent ohmic contact
) to obtain curve 2.
Note that the zero of current is at the top of the
figure. At zero voltage, a current of 120mA flows
through the tandem cell, but to get a current
from the (illuminated) single cell (curve 2), a
voltage has to be provided externally. The
efficiency of the cell is calculated from curve 1
assuming that energy 1.23 eV is associated with
each hydrogen molecule.
-
made in
nding less expensive catalysts for this purpose.) The platinumdeposition is
usually done by electrolytic deposition from a solution containing platinum ions.
These conditions cannot usually all be satis
ed at once without inserting an external
voltage, which has been done in the present example by the extra PN junction. A
survey of the energy levels is given in the next
gure.
According to the compilation of Figure 9.4 [112], GaAs, GaP, and TiO
2
are suitable
to act as photocathodes to release hydrogen fromwater, in that their conduction band
edges all lie above zero on the NHE, normal hydrogen electrode, scale.
9.3.2
Possibility of a Mass Production Tandem Cell Water-Splitting Device
Shown in Figure 9.5 is a schematic of a potentially low-cost tandem device that
achieves direct cleavage of water into hydrogen and oxygen by visible light. This is
based on two photosystems in series (tandem), with electron
flows as shown in the
figure. The top cell (exposed to light) is a thin
lmof tungsten trioxide that absorbs the
blue portion of the solar spectrum. The valence band holes decompose water directly
to oxygen. The conduction band electrons are fed into the second photosystem, the
dye-sensitized nanocrystalline TiO
2
cell. This is placed directly under the tungsten
trioxide film and captures the green and red part of the solar spectrum. The
photoelectrons in the conduction band of the titania reduce water to produce
hydrogen gas.