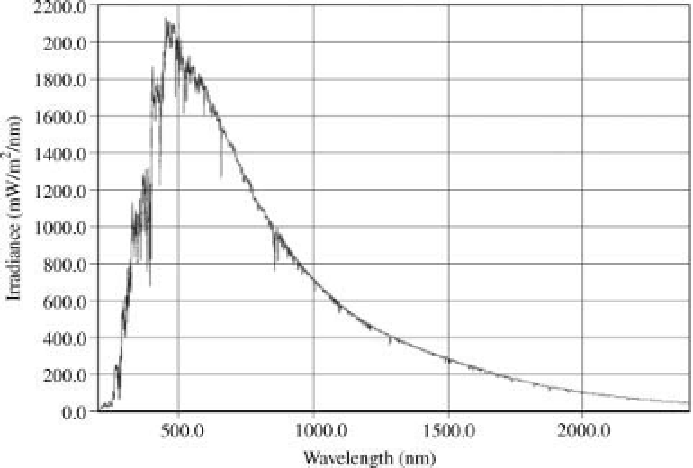
Environmental Engineering Reference
In-Depth Information
should be close to 1366W/m
2
. Note that the
peak here is close to 486 nm, corresponding to a
black body at 5973 K. The portion of this
spectrumbeyond about 700 nmcannot be seen,
but represents infrared heat radiation [4].
Figure 1.3 Directly measured solar energy
spectrum, from200 to 2400 nm, froma satellite-
carried spectrometer just above the earth
s
atmosphere. The units are related to energy,
mW/m
2
nm, and the area under this curve
obtained in the near vacuum above the earths atmosphere. The curve closely
ts the
Planck radiation law,
3
c
3
1
u
ðnÞ¼½
8
p
h
n
=
½
exp
ð
h
n=
k
B
T
Þ
1
;
ð
1
:
1
Þ
10
23
J/K is Boltzmanns constant, and the
Kelvin temperatureT T is 5973 K. This is the Planck thermal energy density, units Joules
per (Hzm
3
), describing the spectrum of black body radiation as a function of the
frequency
n
in Hertz. Equation 1.1 is the product of the number of electromagnetic
modes per Hertz and per cubic meter at frequency
n
, the energy per mode, and the
chance that themode is occupied. The power density is obtained bymultiplying by c/4,
where c
10
34
Js, k
B
¼
where h
¼
6.6
1.38
10
8
m/s is the speed of light. The Planck function is alternatively
expressed in terms of wavelength through the relation
n¼
¼
2.998
c/
l
.
Integrating this energy density over frequency and multiplying by c/4 leads to the
Stefan
-
Boltzmann law for the radiation energy per unit time and per unit area froma
surface at temperature T, which is
5
k
B
4
¼ s
SB
T
4
15 h
3
c
2
10
8
W
m
2
K
4
dU
=
dt
¼
Uc
=
4
; s
SB
¼
2
p
=ð
Þ¼
5
:
67
=
:
ð
1
:
2
Þ
The wavelength distribution of black body radiation peaks at wavelength
l
m
such
that
l
m
T
¼
constant
¼
2.9mmK. The value of
l
m
¼
486 nm for the solar spectrum