Environmental Engineering Reference
In-Depth Information
6.5
Dye-Sensitized Solar Cells
Another potentially inexpensive formof solar cell is the dye-sensitized solar cell. This
approach is based on an absorber that is partly titanium oxide, TiO
2
in its anatase
tetragonal form, and partly dye molecules. Anatase can be prepared in a nanoporous
form (sometimes, described as mesoporous, althoughmicroscopy shows particles of
10
80 nm diameter) by various procedures, one described as hydrothermal proces-
sing of a TiO
2
colloid.
This oxide has a bandgap of 3.2 eV, which means that the maximum light
wavelength absorbed is (1240 eVnm)/(3.2 eV)
-
388 nm. Thus, only a small part at
the UV end of the solar spectrum is absorbed. Spectacular extension of the light
absorption range to at least 700 nmhas been demonstrated by coating (sensitizing) the
nanoporous anatase with dyes. The dyes have a relatively low speci
c absorption, so
that a thick layer of dye is needed, each dye being in intimate contact with the anatase
surface so that a photoelectron can reliably be transferred to the conducting anatase.
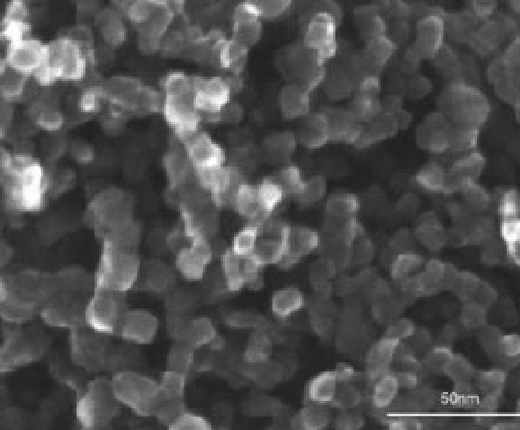
An image is shown in the next
figure of a nanoporous
film, suitable for dye
sensitization.
The hydrothermal deposition procedure for the
film in Figure 6.16 started with
hydrolysis of a titanium isopropoxide precursor and terminated with screen printing
and
firing of the semiconductor layer on a conductive transparent substrate. A
conceptual schematic of a dye-sensitized solar cell based on nanoporous anatase is
shown in Figure 6.17.
¼
Figure 6.16 Scanning electron microscope
image [82] of nanoporous anatase (tetragonal
TiO
2
) film prepared from a hydrothermally
processed TiO
2
colloid. Note scale bar, which
indicates particles about 10
80 nm in diameter.
In dye-sensitized solar cells, dye must fully
penetrate such a deposit, whichmay be 10 mmin
depth, coating each anatase particle [82].
-