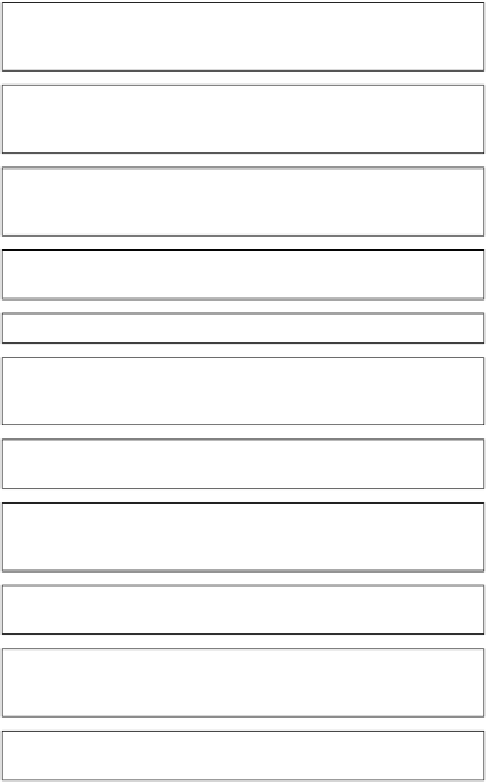
Chemistry Reference
In-Depth Information
Build database of available compounds
In-house and commercially available compounds from
preferred vendors
Elimination of toxic & protein reactive compounds
Apply published and in-house filters for toxic & reactive
moieties in addition to those that lead to screening artifacts
Solubility filter
Select only those compounds with predicted aqueous
solubility
≥
1mM
Clog
P
filter
0-3; average 1.6
H-bond donors, H-bond acceptors, rotatable bonds filter
Feature count
The sum of HBA, HBD and No. of aromatic (heteroaromatic)
rings to be in range 4-7
MW filter
150-350Da; average 247Da
Diversity
Fragments selected for optimal diversity using UNITY
fingerprints
Final selection by medicinal chemists
Each fragment is visually inspected by a medicinal chemist
Analytical QC
Analysis of every fragment by LC-MS and only compounds
with confirmed identity and purity >85% accepted
Store & operate library as dynamically as feasible adding
novel fragments as they become available
Figure 3.3
Process
for
the
assembly
and use of
Evotec's
fragment
library
for
high-concentration biochemical screening.
Second, we analysed such compound repositories with respect to the frequency of
functional groups and scaffolds most abundant in known drugs, as published by Murcko's
group at Vertex.
[
44, 55
]
For example, the two most abundant pairs of functional groups,
carbonyl plus carbonyl and carbonyl plus methyl, occur in 13% and 10% of the 5090
known drugs with assigned therapeutic classes in the Comprehensive Medicinal Chemistry
(CMC) database, respectively. By using 2D substructure filters, we prioritised compound
repositories towards similar percentages of functional groups and scaffolds by filtering out
over-represented groups and scaffolds. This drug-likeness prioritisation was repeated at the
end of the filtering process.