Chemistry Reference
In-Depth Information
11.8 Identification and Optimization of Extended
Dihydroisocytosines
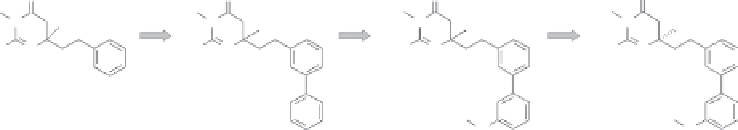
We sought to merge the SAR between the isocytosine and the dihydroisocytosine series.
Compound
18
contains the isocytosine core from
17
but has the extended liker as in
5
(Figure 11.14). The binding affinity of
18
was stronger than that for
5
or
17
and it maintained
high ligand efficiency. A focused library of approximately 15 compounds was prepared
based on
18
to explore various phenyl ring substitutions. The best compound to emerge was
the biphenyl analog
19
. A second focused library of about 15 compounds was constructed
to evaluate substitution at the peripheral phenyl ring and this led to the identification of
20
. After chiral resolution, we identified
21
as the highest affinity inhibitor yet seen. The
absolute configuration of
21
, and also of all other stereochemically defined compounds,
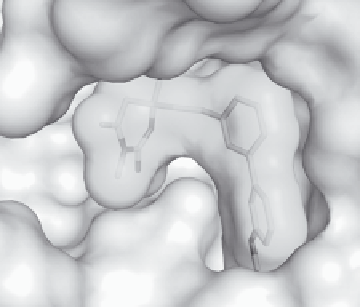
was determined by crystallography (Figure 11.15).
[
42
]
O
O
O
O
Chiral
resolution
N
N
N
N
H
2
N
H
2
N
N
H
2
N
N
H
2
N
N
N
O
O
18
IC
50
34
19
IC
50
2
20
IC
50
0.2
21
IC
50
80 nM
LE
=
0.37
m
m
m
m
LE = 0.34
M
LE
=
0.32
M
LE
=
0.35
Figure 11.14
Combining elements from
5
and
17
led to
18
. Two cycles of focused library
generation led to the identification of
20
. Chiral resolution of this led to
21
as the highest
affinity compound yet identified in our program.
Flap region
S
2
pocket
O
S1 pocket
N
H
N
O
N
H
H
O
Asp228
O
O
S3 pocket
O
Asp32
Figure 11.15
Left: X-ray structure of
21
complexed to BACE. Right: schematic illustration of
the crystallographic analysis of the
9
-BACE complex showing key binding interactions.