Chemistry Reference
In-Depth Information
molecular weight of 127.1 Da, is a primary selection, and is in a cluster (bucket) of 16
members. There are 10 vendors that offer this molecule, with Chem-Impex offering it
in 5 g quantities.
[
48
]
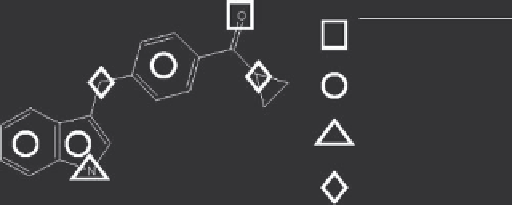
The chemical properties are displayed in the upper right graphical
window, where the number of acceptors, anions, aromatic centroids, cations, donors, polar,
saturated hydrocarbons and unsaturated hydrocarbons are shown. The chemical properties
of an example molecule containing one acceptor, one donor, two saturated hydrocarbons
and three aromatic centroids is shown in Figure 9.18 for illustration. Each fragment in the
database is characterized in a similar manner with the results depicted in Figure 9.17. In this
figure, the vertical axis of the upper right graph, refers to the number of bonds the property
resides from the reference atom, the primary nitrogen in the present example of amine
fragments. The last pair of columns on this graphical object displays the distribution of
synthon molecular weights. For each property, there are two columns, displayed as circles
and diamonds, which represent the properties of the universe of 5000 molecules and the 25
primary selections, respectively. The numbers enclosed by the circle or diamond indicate
the number of molecules with the associated property/bond count. Each circle and diamond
is also dynamically linked to the film strip, so by clicking on any of these graphical objects,
the film strip will be populated with the molecules contributing to its count. The reverse is
also true: by clicking on a molecule in the film strip, as is the case for the third molecule,
MFCD06247767, the properties in the graphic window corresponding to this molecule are
outlined in a heavier line. In this way, the structural and chemical space of all fragments
may be quickly navigated. This visual approach coupled with the interactivity of the web
page allows users to better optimize the fragment selection process.
Chemical Property
acceptor
aromatic centroid
donor
sat hydrocarbon
Figure 9.18
The chemical properties of an example synthon containing one acceptor, one
donor, two saturated hydrocarbons and three aromatic centroids.
Once the chemistry, scaffold and fragments have been determined, the Library Enumer-
ation (LibE) component of VLTK will fully enumerate the library in either 2D or 3D.
[
43
]
In many cases, 2D enumeration will be sufficient for analysis since many of the physical
properties and even QSAR models often rely only on connection tables. However, in the
present example a complete 3D enumeration is required for subsequent docking and scoring
in the BACE-1 active site. To preserve the position of the scaffold shown in Figure 9.15,
with respect to the active site, the 3D coordinates are written directly into a molecular
structure file that is then read into LibE. All fragments are added to the scaffold without
changing the scaffold position. Molecules of the fully enumerated molecule will have the
correct position and orientation for initiating docking and scoring.